|
 |
- Search
| Obstet Gynecol Sci > Volume 66(5); 2023 > Article |
|
Abstract
Nowadays, polycystic ovary syndrome (PCOS) and cognitive dysfunction are major health problems among female. This narrative review aimed to investigate cognitive dysfunction in female with PCOS. English and Persian articles published in PubMed, Scopus, Web of Science, Google Scholar, PsycINFO, Scientific Information Database, and Cochrane Database of Systematic Reviews until May 2022 were searched. Sixteen studies involving 850 female with PCOS and 974 controls were assessed. In these studies, the association between biochemical factors and symptoms of PCOS and memory, attention, executive functioning, information processing speed, and visuospatial skills was evaluated. The literature review revealed the possible cognitive changes in female with PCOS. This study summarized the different aspects of cognitive function in female with PCOS due to medication, psychological problems (mood disorders caused by disease symptoms and complications), and biochemical markers, such as metabolic and sex hormone abnormalities. Considering the existing scientific gap regarding the possibility of cognitive complications in female with PCOS, further biological studies should be conducted to evaluate the potential mechanisms involved.
Polycystic ovary syndrome (PCOS) is a common health problem among female of childbearing age worldwide [1-3]. Disorders and complications associated with PCOS impose significant psychological and economic burdens on different people in different countries [4-7]. Several studies have indicated various aspects of cognitive dysfunction in patients with PCOS [8-10].
Cognitive functioning refers to multiple mental abilities, including learning, thinking, reasoning, remembering, problem solving, decision making, and attention [11]; dementia is a progressive or long-lasting condition that leads to cognitive changes [12,13]. By 2019, approximately 50 million people had dementia worldwide, with an incidence of new cases every three seconds [14,15]. As a cognitive disorder, dementia is the fifth-leading cause of mortality globally [16]. A study on 70 postmenopausal female with PCOS and 140 control female showed that white matter lesions and silent cerebral infarcts increased in female with PCOS [17]. In another study, PCOS was associated with a remarkable reduction in diffusion along the main axis of white matter fibers and cognitive function was diminished compared to the control group [8].
Cognitive function is strongly related to age [18], Although cognitive impairment is common after menopause, it has been proposed that cardiovascular risk factors during the premenopausal period might adversely affect cognitive function [19]. In line with this study, the results of a study on healthy young female of reproductive age showed a possible association between obesity and cognitive function [20]. The effect of age on the progressive disease course in female with PCOS is contradictory, and it has been observed that with increasing age in these female, menstrual cycles become regular because the production of androgens decreases with increasing age [21-23]. Since female with PCOS are at an increased risk of developing cardiovascular risk factors before menopause, it has been hypothesized that they may also be at risk of cognitive dysfunction during the premenopausal period.
Overall, narrative review papers are comprehensive and cover a wide range of topics on a specific subject [24,25]. A narrative review by consolidation and summation of prior studies could develop new conceptions [26]. As PCOS and cognitive disorders are important health problems worldwide, this study was conducted as a narrative review to discuss cognitive dysfunction in female with PCOS, which may pave the way for further extensive research investigating the correlation between the two conditions.
This narrative review was conducted to survey cognitive function in female with PCOS. Articles published in PubMed, Scopus, Web of Science, Google Scholar, PsycINFO, the Scientific Information Database, and the Cochrane Database of systematic reviews dated until to May 2022 were searched. There were no time limitations in the search for articles. Articles in English and Persian were searched using the following keywords and their derivatives: polycystic ovary syndrome, PCOS, acne, hirsutism, obesity, drug, brain function, insulin resistance, metabolic syndrome, hormones, dementia, mild cognitive impairment, Alzheimer’s disease (AD), cognitive function, cognitive impairment, cognition, cognitive decline, cognitive health, depression, anxiety, and androgen.
The findings of this study are reported in the subject matter section. This review was based on a standard checklist for quality assessment of narrative review articles [27]. The inclusion criteria were observational and experimental studies and reviews and articles that provided related content and objectives of this review paper. Articles containing duplicate content or those that provided content outside the scope of this study were excluded. To ensure comprehensiveness of the search, the list of references used in the articles was examined. The participants in the included studies were female with PCOS, without age limitations. The outcomes of these studies were measures of cognitive change.
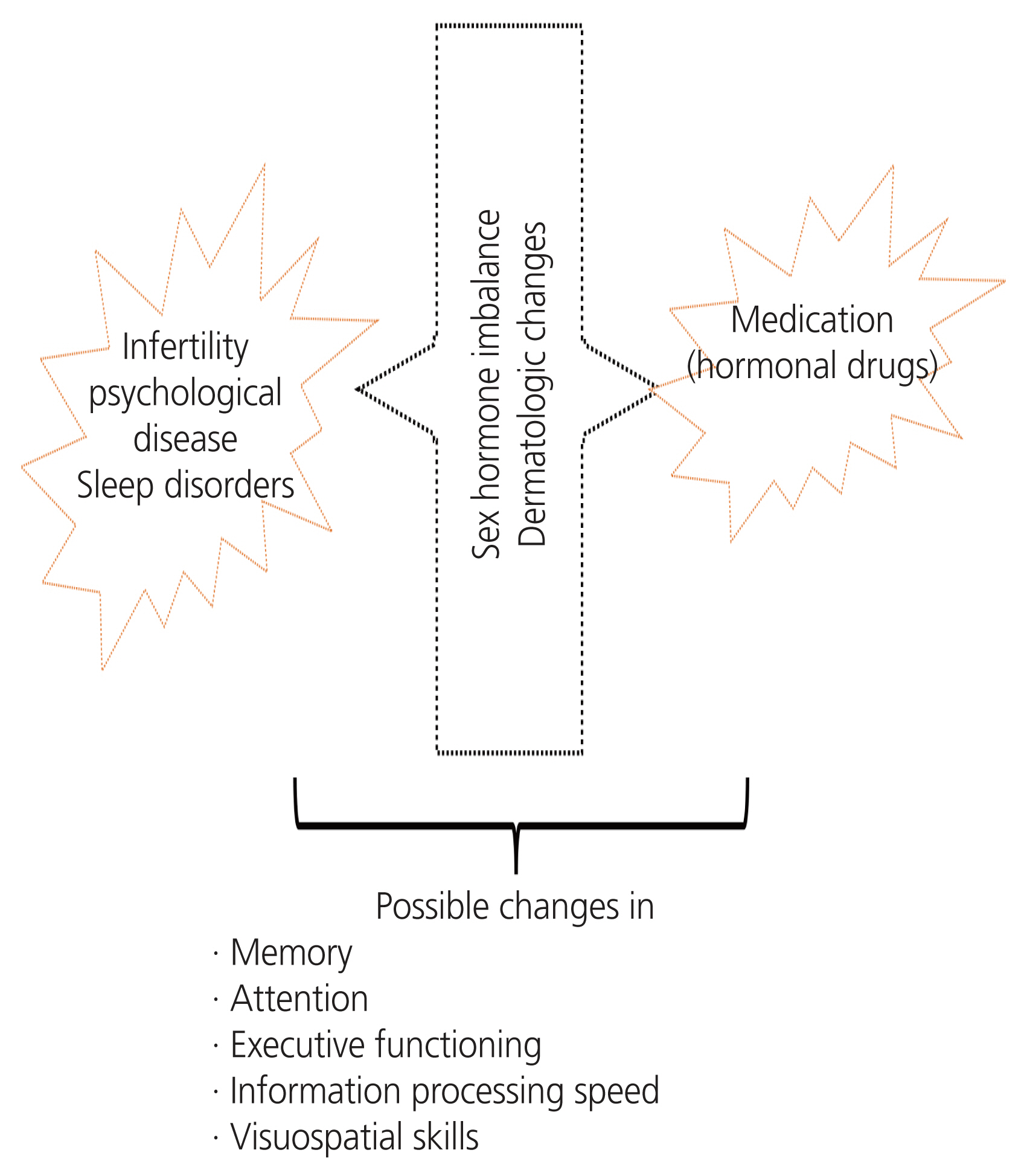
This review assessed 16 studies involving 850 female with PCOS and 974 controls. In these studies, the associations between biochemical factors and symptoms of PCOS with memory, attention, executive functioning, information processing speed, and visuospatial skills were evaluated. This study summarized the different aspects of cognitive function in female with PCOS due to medication, psychological problems (mood disorders caused by disease symptoms and complications), and biochemical markers such as metabolic and sex hormone abnormalities. Table 1 presents the characteristics of these studies. Fig. 1 shows possible cognitive changes in female with PCOS.
Female with PCOS suffer from hormonal disorders such as increased androgen levels, disrupted luteinizing hormone (LH)/follicle stimulating hormone (FSH) ratio, and higher estrogen levels [28]. A recent review has described potential neuroendocrine- and brain-specific mechanisms in the development of PCOS [29]. Research comparing cognitive functions among boys and girls has shown that the cognitive function of girls in the post-ovulation phase is similar to that of boys. In contrast, their cognitive function before the ovulation phase differs from that of boys [30]. Testosterone is a sex hormone that plays an essential role in the cognitive function of both male and female [31]. It has been reported that people with high testosterone levels perform better on 3 dimensions (3D) mental rotation cognitive tasks [32]. Rees et al. [33] conducted a study on 18 female with PCOS and 18 healthy controls, and found a significant correlation between blood testosterone levels, axial diffusivity (AD) and fractional anisotropy. However, no such correlation was observed in the control group [33]. Through two types of receptors (estrogen receptors alpha and estrogen receptors beta), estrogen has a wide range of functions in different parts of the brain (cortex, hippocampus, hypothalamus, sensory ganglia). Thus, it is effective in improving cognitive and motor functions and emotional states [34]. However, a conflicting study indicated that the levels of different sex hormones during the menstrual cycle were not related to female’s cognitive function [35]. In this regard, the results of another study showed that high levels of progesterone were associated with increased amygdala reactions and higher levels of emotional memory [36]. In fact, during the luteal phase, an increased response of the amygdala to negative emotional stimuli was observed in this study [36]. The amygdala plays an important role in cognitive function [37].
In a study of 135 female with PCOS and 322 healthy controls, Barnard et al. [10] examined the cognitive function for both groups, in which female with PCOS had impairments in terms of speed, accuracy, and reaction to the role of words. In an investigation on 69 female with PCOS and 41 healthy controls, visual-spatial cognition was measured using a 3D mental rotation tool, and its relationship with the level of sex hormones was examined; female with PCOS showed a higher score on the 3D mental rotation score task than those in the control group. In addition, the level of testosterone in the circulation had a significantly positive correlation with the 3D mental rotation score, but the level of estradiol had an inversely significant correlation with the 3D mental rotation score [38]. However, it should be noted that the electrochemiluminescence method used for the measurement of testosterone in this study might have low sensitivity for the accuracy of detection and may have affected the results of this study. It is also highlighted that this relationship might stem from prenatal androgenization in PCOS. Franik et al. [8] study on 55 female with PCOS indicated an inversely significant relationship between free testosterone (fT) levels and verbal psychomotor speed, suggesting that better verbal psychomotor speed was observed among female with higher fT. Additionally, high androstenedione levels are were associated with a poor level of executive function [8]. It has been showed that androgens act as regulators of amyloid-beta peptide (Aβ) production [39] and Aβ accumulation in the brain is proposed to be an early toxic event in the cognitive alterations such as dementia [40].
In a study involving 22 female with PCOS and 22 healthy female, Schattmann found that verbal memory and fluency, visuospatial working memory, and manual dexterity were worse in female with PCOS than in controls [41]. In this study, higher fT values were associated with lower verbal fluency. In contrast, Kara et al. [42] in their study among 70 adolescents with PCOS, found that mindfulness was not associated with high serum androgen levels. Evidence also suggests that PCOS can affect the function of parts of the brain, reducing face processing and visuospatial working memory. Serum LH levels and LH/FSH ratio are associated with reduced right frontal lobe function in female with PCOS [43]. A possible underlying mechanism is the Aβ. In cases with LH abnormalities, LH promotes the amyloidogenic pathway, eventually leading to cognitive changes [44]. Cognitive function relies on neuronal network interactions and alterations in the hormonal system (steroidal, sexual, and maternal) and can influence cognitive function [45]. There are four phenotypes of PCOS with different hormonal characteristics [46]. It is important to note that the potential effects of hormonal malfunction on cognitive function in female with PCOS may differ between different phenotypes. Hence, the results of these studies remain unconfirmed, and more robust studies should confirm the association between hormonal abnormalities and potential cognitive changes in female with PCOS.
Studies have indicated that female with PCOS are approximately three and five times more likely to develop depressive and anxiety symptoms, respectively. Those with higher body mass index are also at greater risk of depression and anxiety symptoms [47]. In a study of 53 patients with PCOS and 54 unaffected female, Sukhapure [48] reported that the patient group experienced more depression and cognitive impairment than the other female. High testosterone levels are associated with mood disorders and cognitive dysfunction in verbal and visuospatial learning, memory, psychomotor speed, and emotion processing. Anti-androgen therapy has been associated with improved psychomotor speed and various aspects of emotional processing, and mood improvement is related to improved psychomotor speed, attention, and executive function [48]. A case-control study of 53 female with PCOS and 50 controls aimed at examining the relationship between depression and anxiety and cognitive function in female with PCOS compared with a healthy group indicated that although the PCOS group was more depressed and anxious than the healthy group, there was no significant difference in cognitive functioning between the two groups [49]. Cognitive well-being is closely linked with psychological well-being [50]. Chronic exposure to higher levels of glucocorticoids (GCs) could adversely affect the brain regions responsible for memory and emotions; hence, GCs could contribute to both mental health and cognitive dysfunction [51]. Taken together, further investigations of psychological consultations and their possible impact on cognitive function in female with PCOS are required.
Female with PCOS may also develop sleep disorders. Obstructive sleep apnea (OSA) is a common sleep disorder in female with PCOS [52,53] that occurs when the upper airway is partially or entirely blocked, leading to a pause in breathing during sleep [54]. Obesity is a leading risk factor for PCOS and OSA [53]. Cognitive deficits have also been observed in patients with OSA [55]. Subsequent intermittent brain hypoxia with recurrent apneic pauses in patients with OSA can cause structural and cerebrovascular damage to the brain, as well as cognitive impairment [56]. The pathophysiological mechanisms underlying the association between OSA and cognitive impairment include blood-brain barrier disruption, hemodynamic and vascular changes, and intermittent hypoxemia [57]. Furthermore, it is well documented that among female, alterations in estradiol and progesterone levels may be associated with sleep pattern abnormalities and memory problems [58]. Hence, female with PCOS who are at high risk of hormonal abnormalities and sleep disorders might be more prone to cognitive changes.
PCOS causes dermatological problems, and acne is a symptom of hyperandrogenism [59]. Deveci et al. [60] studied 66 female with acne and 47 controls to assess their cognitive abilities, social phobias, anxiety, and depression. This study showed poorer function in the acne group compared to the control group in terms of cognitive tests assessing attention, verbal episodic memory, working memory, and phonemic verbal mastery [60]. Mehrabadi et al. [61] conducted a study involving 45 female with PCOS and 45 healthy female to investigate the relationship between acne and cognitive function. A global grading scale for acne was used to examine acne severity and the Montreal cognitive assessment (MoCA) test was used to evaluate cognitive function. The cognitive performance score in the case group was significantly lower than in the control group; however, there was no significant relationship between acne severity and cognitive function [61]. According to the evidence, acne may lead to psychological issues [62,63], and psychological problems may lead to cognitive impairment [64]. The heightened level of psychosocial stress in individuals with psychocutaneous disease affects the hypothalamic-pituitary-axis and contributes to a vicious cycle of worsening disease symptoms [65]. It should be noted that in a study among adolescents with acne, the treatment approach resulted in improvement in neurocognitive functions [66]. Therefore, psychocutaneous therapy may affect cognitive wellbeing.
However, there is insufficient data on the impact of acne on cognitive function among female with different PCOS phenotypes.
Metabolic disorders and metabolic syndromes are long-term complications of PCOS and are commonly observed in various PCOS phenotypes [67,68]. Metabolic syndromes can also affect cognitive performance [69]. Lujan and Mergler [70] studied 79 female with PCOS and 40 healthy female, and assessed their cognitive function. The findings showed that female with PCOS have poorer manual dexterity and spatial ability. In the present study, metabolic and reproductive factors predicted cognitive function in female with PCOS [70].
Diabetes and insulin resistance have been identified as mechanisms responsible for cognitive dysfunction. In this regard, a study of Danish female with type 2 diabetes showed that abnormalities in fasting blood sugar were associated with a 44% increase in the likelihood of developing cognitive dysfunction [71]. A study of 353 German subjects with diabetes (type 1 or 2) found that the number of people with verbal memory disorders was significantly higher in patients with type 2 diabetes than in those with type 1 diabetes [72]. Rees et al. [33] conducted a study involving 18 female with PCOS and 18 healthy subjects. They showed that increased insulin resistance was associated with reduced AD in the control group but increased AD in those with PCOS [33].
The research results by Castellano et al. [73] indicated that in female with PCOS, the cerebral metabolic rate of glucose (CMRglu) was 9-14% lower in the frontal, parietal, and temporal cortices. In addition, CMRglu had an inversely significant relationship with homeostatic model assessment-insulin resistance, which is similar to the pattern observed in aging and Alzheimer’s disease [73]. The role of insulin in the brain of healthy individuals includes the central modulation of metabolism, boosting or regulating memory, and other cognitive and emotional functions. Insulin resistance in the brain can be defined as the failure of brain cells to respond to insulin, which typically disrupts synaptic, metabolic, and immune responses. The findings of a study on 40 female with PCOS and 40 healthy female aimed at examining cognitive dysfunction showed that visual-spatial ability was lower in female with PCOS relative to healthy female and that decreasing visual-spatial cognition was related to hemoglobin A1c levels [74], which are associated with cognitive function [75,76]. Insulin resistance, obesity, hyperlipidemia, and disorders of homocysteine metabolism may be major factors in the development of cognitive impairment in female with PCOS, rather than androgens [77]. A case-control study of 79 female with PCOS and 40 controls revealed that female with PCOS had poorer spatial abilities and manual dexterity scores. The metabolic status and fertility in female with PCOS were predictive of manual dexterity [70]. More robust observational studies and randomised controlled trials should address the beneficial effects of controlling metabolic risk factors on cognitive function in female with PCOS in the long term.
Obesity and being overweight are frequent problems in female with PCOS [78]. Cook et al. [79] conducted a study to investigate the relationship between obesity and cognitive impairment in Arab female aged 18-35. This study showed that obese female had significantly lower cognitive functioning and attention, indicating an early disruption of cognitive functioning in obese female [79]. Another study of 110 Chinese male and female aged over 60 years revealed a 1.53-fold increase in cognitive dysfunction with obesity [80]. The mechanism underlying the association between obesity and cognitive dysfunction is unclear; however, it may be related to cerebral atrophy, cerebrovascular disorders, and systemic and central inflammation [81]. Some evidence supports the adverse effects of obesity on cognitive function in female, but the longitudinal impact of obesity and weight gain in female with PCOS is unclear.
A recent study demonstrated that female with PCOS have brain abnormalities at the regional and network levels, and these alterations are associated with serum hormones and cognitive function [82]. Moreover, a study conducted by Lai et al. [43] on 21 female aged 18-45 with newly diagnosed PCOS, which aimed to investigate the effect of PCOS on brain activity and explore the relationship between brain activity and sex hormone levels in female with PCOS demonstrated that high levels of LH could induce changes in the activity of brain regions responsible for visuospatial working memor, face processing, and episodic memory. In another study, a significant difference was found in the mean grades of acne, hirsutism, total testosterone, free androgen index, depression, anxiety, MoCA as well as in the domains of visual-spatial ability, executive function, and attention in the PCOS group [83]. In addition, higher free testosterone levels in female with PCOS were associated with poorer cognitive function, specifically psychomotor speed and visuospatial learning [84].
Complications or diseases associated with metabolic syndromes, such as hypertension, can also be associated with cognitive impairment. High blood pressure is another complication of PCOS [85,86]. Studies have demonstrated that hypertension disrupts the structure and function of the cerebral blood vessels, leading to ischemic damage to the white matter involved in cognitive function, which may be related to Alzheimer’s pathology. There is strong evidence of the destructive effect of hypertension on cognitive function in middle-aged individuals. However, the effect of high blood pressure on cognitive functioning at a later age is less obvious. Observational studies have unveiled the cumulative impact of high blood pressure on cerebrovascular damage. Nevertheless, there is no evidence from clinical trials indicating that antihypertensive therapy can improve cognitive function [87]. The cross-sectional outcome of a review examining the relationship between high blood pressure and cognitive functioning showed a correlation between blood pressure and cognition, revealing conflicting connections with positive, negative, and J- and U-shaped relationships. Most longitudinal studies have reported that hypertension is associated with diminished cognitive function. A few randomized controlled trials have revealed the heterogeneous effects of lowering blood pressure on cognitive function, suggesting a complex relationship between blood pressure and cognitive function consistent with biological mechanisms [88]. No study has dealt with the relationship between cognitive function and high blood pressure in female with PCOS.
Evidence suggests that a history of fertility is a factor that influences the cognitive status of female in later life [89]; therefore, several researchers have found a U-shaped relationship between the number of children and cognitive function in old age [90]. Infertility is a common complication of PCOS [91]. A study involving 830 postmenopausal female in California found that fertility history was linked to cognitive function. Delayed pregnancy after 35 years of age was significantly positively correlated with verbal memory. According to the results of this study, female’s reproductive duration has a significantly positive relationship with cognitive function [92]. Meanwhile, the results of a cohort study on postmenopausal female in Latin America and China showed that dementia was not significantly correlated with nulliparity [93]. Another study of 18 female with PCOS and 18 healthy female found partial impairment of cognitive function in female with PCOS [9]. Female’s fertility status is an indicator of estrogen exposure. Cumulative estrogen exposure during reproductive years in female can affect cognitive function [94]. However in female with PCOS, abnormalities in estrogen and estrogen receptors have been observed more frequently [95]. Female with higher parity numbers have a larger gray matter volume, which is linked to cognitive function [96]. Treatment of infertility might influence the cognitive function of female owing to the manipulation of sex hormone levels; however, a study by Leeners et al. [97] did not support the association between high estradiol levels resulting from fertility treatment and cognitive function.
Further studies are required to compare the different aspects of infertility and the number of children in female with PCOS. Additional studies are needed to clarify how many years of infertility could affect cognitive function.
Metformin and hormone therapy, including oral contraceptives, are treatment options for female with PCOS [98]. A systematic review that examined the findings of 22 studies on the effects of oral contraceptives on cognition showed that contraceptive pills are associated with improved verbal memory. However, depending on the androgenicity, the use of this type of medication and its containing progesterone content could positively or negatively affect visual-spatial cognition [99]. A study of 830 postmenopausal female in California found that hormonal contraceptive pills significantly affected cognitive functioning and verbal memory. Taking contraceptive pills for more than 10 years boosts verbal memory and executive function [60]. Specialists usually perform symptomatic treatment for PCOS. For example, acne is one of the symptoms usually subject to symptomatic treatment. In a study performed on 55 adolescents; 32 adolescents received antibiotics for acne treatment and another group of 59 received isotretinoin, which indicated improved cognitive function in the group taking isotretinoin [100]. There are no studies of this type in female with PCOS in this respect.
Soleman et al. [101] conducted a study on 40 female with PCOS and hyperandrogenism and 20 healthy female. In this study, cyproterone acetate (25 mg/dL) combined with hormonal oral contraceptives (35 mg ethinyl E2/d and 2 mg cyproterone acetate, orally) was administered; the intervention group did not receive such treatment, and working memory (speed and accuracy of performance) was measured. The findings of this study revealed that in female with PCOS, all memory conditions related to the parietal (superior and inferior) and temporal (superior) lobes were different from those of the control group. However, there was no difference in performance [101].
A randomized trial was conducted to investigate the effects of hormone treatment on fT levels and cognitive function in female with PCOS. Hormone therapy in female with PCOS significantly reduced their fT levels but did not affect manual dexterity, perceptual speed, visuospatial ability, or verbal memory. However, improved verbal dominance testing scores were observed in female with PCOS compared to their pre-treatment scores. These findings suggest that changes in fT levels do not significantly affect the cognitive function in patients with PCOS [102]. Another study of 68 female with PCOS and 27 healthy female found that female with PCOS had lower MoCA scores. However, cognition improved after three months of metformin treatment [103].
The limitation of this study is that because of the lack of evidence, we could not determine the exact role of symptoms and markers of PCOS and its related medications in the development of cognitive changes in PCOS patients.
In recent years, the incidence of PCOS has increased, resulting in higher disability-adjusted life-years [104]. There are different therapeutic approaches for management of PCOS [105-107]. The underlying pathophysiological mechanisms contributing to the development of PCOS are multifactorial; PCOS is more than one reproductive disorder [108]. As previously mentioned, poor cognitive performance has been observed in individuals with PCOS. However, the exact pathophysiological mechanisms underlying the association between PCOS and cognitive dysfunction remain unknown. PCOS and cognitive dysfunction have been proposed to share common risk factors. PCOS symptoms and comorbidities, such as infertility, appearance changes, cosmetic problems, metabolic disturbances, and sleep problems, could pose the greatest threat to psychological distress in females with PCOS, while psychological distress and cognitive dysfunction are linked together [109,110]. Along with psychological distress, metabolic conditions can contribute to the loss of cognitive function [110]. Moreover, metabolic impairment can affect different aspects of cognitive function, such as executive functioning, visuospatial abilities, and processing speed [111]. Insulin resistance, a prevalent metabolic perturbation in PCOS [108], connects metabolic syndromes with cognitive impairment [112]. Notably, metabolic disturbances caused by PCOS have discernible effects on metabolic tissues such as the brain [108]. Moreover, brain structure is affected by sex hormone fluctuations. Insulin resistance and hyperandrogenism may result in structural and functional brain disturbances [113].
Despite the seriousness of cognitive impairment in female with PCOS, evidence in this area of research is limited. Considering the existing scientific gap concerning the possibility of cognitive complications in female with PCOS, more biological studies should be conducted to evaluate the potential mechanisms involved. Further studies are required to understand whether there is a relationship between the symptoms and comorbidities of PCOS and poor cognitive function. Overall, recent advances in understanding the etiology and risk factors of cognitive changes in female with PCOS are not comprehensive, and the lack of previous studies in this field represents a gap in knowledge. Identifying similar mechanisms may play a pivotal role in identifying predictive biomarkers of cognitive dysfunction in female with PCOS. Moreover, future research needs to cover the neurobiological and neuroendocrinological aspects of the link between PCOS and cognitive dysfunction.
Evidence shows that disorders related to glucose and insulin may result in poor cognitive function [114]. On the other hand, psychological distress also could affect cognitive function. Female with PCOS may develop either or both. PCOS is complex and heterogeneous; besides the visible reproductive and dermatological aspects of PCOS, silent features should also be considered in the management of patients. Therefore, best management is achieved by a multidisciplinary care team [115]. Comprehensive psychological assessments and emotional, psychological, and neurocognitive support are essential for the well-being of female with PCOS [116]. Recent guidelines for assessing and managing PCOS have emphasized the critical importance of screening, diagnostic assessment, and treatment for emotional well-being in female with PCOS [117]. Different approaches have been suggested to improve the psychological aspects of PCOS, such as cognitive behavioral therapy, mindfulness, relaxation, guided imagery, and lifestyle modifications [118].
Metabolic screening and brief psychological and cognitive screening in female with PCOS should be considered critical parts of their health care. The ultimate goal of this field is to promote well-being, improve the quality of life of female with PCOS, and reduce the burden of life-threatening disorders, such as dementia and AD.
In the coming years, the increasing prevalence of PCOS and cognitive impairment will become an interesting and key field of research. Future scientific research should focus on predictive biomarkers of cognitive changes in female with PCOS. It is predicted that more observational studies will be conducted to identify knowledge gaps regarding the link between cognitive changes and PCOS. Moreover, there will be a growth in clinical trials to reduce the development of cognitive changes in female with different phenotypes of PCOS within different age groups (adolescents, adults, and menopause).
Optimal diagnosis and improved management of PCOS by improving metabolic and hormonal disorders and decreasing infertility and psychological complications could reduce the risk of cognitive dysfunction in female with PCOS. Lifestyle interventions such as dietary and exercise interventions, obesity, weight assessment, and behavioral strategies have been suggested as cornerstones in the management of female with PCOS [117]. Nutritional interventions to improve metabolic abnormalities should be advised in female with PCOS. Further clarification on the role of lifestyle modifications in preventing cognitive dysfunction in female with PCOS is necessary. In addition, there will be more studies on the development of molecular tools for diagnosing cognitive changes in female with PCOS and indicating the effect of interventions on reducing cognitive dysfunction or preventing the progression of cognitive changes in female with PCOS over the next 10 years. It is also expected that within the next 5 years, some female with PCOS will undergo cognitive function screening as part of their medical care.
A literature review revealed the possible cognitive changes in female with PCOS. Research has shown that the brain structure is affected by sex hormone fluctuations and metabolic disorders. Therefore, PCOS and cognitive dysfunction may share some common risk factors. Along with significant attention to the fertility aspect of PCOS, a greater focus on cognitive problems in female with PCOS is required.
Notes
Conflict of interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Table 1
Characteristics of studies
| Study | Participants | Design | Clinical and paraclinical assessment | Cognitive domain | MRI | ||||
|---|---|---|---|---|---|---|---|---|---|
| Memory | Attention | Executive functioning | Information processing speed | Visuospatial skills | |||||
| Sukhapure et al. [84] (2022) | PCOS group (n=40), mean age (28.72±7.44) | Cross-sectional study | Hormone | Higher free testosterone levels were associated with poorer performance on measures of psychomotor speed (TCT) | Higher free testosterone levels were associated with poorer performance on measures of visuospatial learning (GMLT) | ||||
| Ranjbar et al. [119] (2020) | PCOS (n=50), control (n=50); mean age: PCOS (25.6±4.8); control: (29.3±5.8) | Cross-sectional study | Mood | The mean scores of spatial memory test were significantly different between PCOS and control group (0.52±0.88 versus 0.39±0.06) | |||||
| Li et al. [82] (2020) | PCOS group (n=41), control (n=41), mean age: PCOS group (25.29±3.15); control group (21.8±2.1) | Cross-sectional study | Hormone, insulin resistance, and triglyceride | PCOS group had longer executive control times | There are some changes in regional and network levels of brain function in female with PCOS | ||||
| Mehrabadi et al. [83] (2020) | PCOS group (n=53); control group (n=50), mean age: PCOS group (28.47±6.27); control group (29.94±6.24) | Cross-sectional study | Hormone | Female with PCOS had lower score in this domain | Female with PCOS had lower score in this domain | Female with PCOS had lower score in this domain | |||
| Barnard et al. [10] (2007) | PCOS group (no AA=112, AA=109); control group (no AA=256, AA=186); mean age: PCOS group (no AA=30±6.45, AA=29±5.66); control group (no AA=31±7.61, AA=26±4.32) | Internet-based study | Hormone mood | Impaired performance in terms of speed and accuracy | |||||
| Barry et al. [38] (2013) | PCOS (n=69) and control (n=41); mean age: PCOS=29 (18-38); control=35 (24-34 yr) | Cross-sectional study | Hormone | Link between visuospatial cognition and sex hormones | |||||
| Castellano et al. [73] (2015) | PCOS (n=7) and control (n=11) mean age: PCOS=24.6±5.9; control=24.0±3.3 | Compared study | Insulin resistance | Lower score in working and episodic memory | 10-17% lower volume of several brain/10% smaller left superior frontal cortex/14% smaller left supramarginal cortex/10% smaller right superior parietal cortex | ||||
| Franik et al. [8] (2019) | 55 female with PCOS; mean age: 24.35±4.164 | Case-control study | Hormones and metabolic | Androstenedione level was associated with worse scores in executive functions | Higher free testosterone correlated with better verbal psychomotor speed | ||||
| Jarrett et al. [74] (2019) | PCOS (n=40) and control (n=40); mean age: PCOS=27±5, control=27±4 | Observational, cross-sectional study | Reproductive and metabolic features | Reduced visuospatial ability | |||||
| Lai et al. [43] (2020) | PCOS (n=21) and control (n=27); mean age: PCOS=25±5, control=21.8±2.1 | Observational, cross-sectional study | Decreased activities of regions responsible for visuospatial working memory | Increased amplitude of low-frequency fluctuation (ALFF) in left inferior temporal gyrus and decreased in left inferior occipital gyrus | |||||
| Lujan and Mergler [70] (2015) | PCOS (n=79) and control (n=40) | Case-control study | Metabolic and reproductive factors predictor of spatial ability | Lower scores on the mental rotation task on spatial ability and manual dexterity | |||||
| Schattmann and Sherwin [102] (2007) | PCOS with HRM group (n=8) and placebo group (n=11); mean age: HRM=26.8±6, placebo=26.6±5.2 | Randomized, placebo-controlled trial | Hormones and mood | Hormone treatment doesn’t change verbal memory | Hormone treatment doesn’t change manual dexterity, or perceptual speed | Hormone treatment doesn’t change visuospatial ability | |||
| Soleman et al. [101] (2016) | 14 female with PCOS and 20 healthy control female/mean age: PCOS=29.3±5.6, control=25.6±6.2 | Longitudinal study/antiandrogenic hormone treatment | Hormonal | Task performance (speed and accuracy) did not differ between the groups | PCOS differed brain activation in the parietal lobe (superior and inferior) and in the temporal lobe (superior) than control female | ||||
| Rees et al. [33] (2016) | PCOS (n=18) and control (n=18)/mean age: PCOS=31±6, control=31±7 | Case-control study | Anthropometric and biochemical measurements | Episodic memory cognitive performance was degraded in patients with PCOS compared with controls |
Intelligence and executive function Cognitive performance was degraded in patients with PCOS compared with controls |
Lower value of AD/higher value of tissue volume fraction testosterone level correlated with microstructural measures | |||
| Sukhapure [48] (2019) | PCOS (n=53) and non-PCOS control (n=54) | Experimental baseline and 12 weeks after antiandrogen treatment | Mood symptoms | Correlation between visuospatial learning and memory and worse mood and anxiety | Improve in attention by improvement in symptoms of mood | Improve in executive function by improvement in symptoms of mood | Verbal and visuospatial learning improvement aftern treatment | ||
| Guoqing et al. [17] (2016) | Control (n=140); mean age: PCOS: 55.04±2.55; control: 55.11±2.66 | Case-control | Hormones metabolic | Higher prevalence of white matter lesions and silent cerebral infarcts | |||||
References
1. Wolf WM, Wattick RA, Kinkade ON, Olfert MD. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health 2018;15:2589.



2. Ding T, Hardiman PJ, Petersen I, Wang FF, Qu F, Baio G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget 2017;8:96351-8.



3. Naz MSG, Tehrani FR, Majd HA, Ahmadi F, Ozgoli G, Fakari FR, et al. The prevalence of polycystic ovary syndromein adolescents: a systematic review andmeta-analysis. Int J Reprod Biomed 2019;17:533-42.


4. Riestenberg C, Jagasia A, Azziz R. Pregnancy-related economic burden of polycystic ovary syndrome (PCOS). Fertil Steril 2019;112:e43.

5. Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab 2005;90:4650-8.


6. Sulaiman MA, Al-Farsi YM, Al-Khaduri MM, Waly MI, Saleh J, Al-Adawi S. Psychological burden among women with polycystic ovarian syndrome in Oman: a case-control study. Int J Womens Health 2017;9:897-904.


7. Saei Ghare Naz M, Ramezani Tehrani F, Behroozi Lak T, Mohammadzadeh F, Nasiri M, Kholosi Badr F, et al. Quality of life and emotional states of depression, anxiety and stress in adolescents with polycystic ovary syndrome: a cross-sectional study. Psychol Res Behav Manag 2020;13:203-9.


8. Franik G, Krysta K, Witkowska A, Dudek A, Krzystanek M, Madej P. The impact of sex hormones and metabolic markers on depressive symptoms and cognitive functioning in PCOS patients. Gynecol Endocrinol 2019;35:965-9.


9. Udiawar M. Cardiometabolic and neuroimaging correlates of cognitive function in polycystic ovary syndrome Cardiff University; 2017 Jul 31 [Epub]. https://orca.cardiff.ac.uk/id/eprint/103000
.
10. Barnard L, Balen AH, Ferriday D, Tiplady B, Dye L. Cognitive functioning in polycystic ovary syndrome. Psychoneuroendocrinology 2007;32:906-14.


11. Fisher GG, Chacon M, Chaffee DS. Theories of Cognitive Aging and Work. In: Baltes BB, Rudolph CW, Zacher H, editors. Work Across the Lifespan. Cambridge (MA): Elsevier Academic Press; 2019. p. 17-45.

12. Vos SJB, van Boxtel MPJ, Schiepers OJG, Deckers K, de Vugt M, Carrière I, et al. Modifiable risk factors for prevention of dementia in midlife, late life and the oldest-old: validation of the LIBRA index. J Alzheimers Dis 2017;58:537-47.


13. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673-734.


14. World Health Organization. Dementia [Internet] Geneva (CH): World Health Organization; c2022 [cited 2022 May 1]. Available from: https://www.who.int/health-topics/dementia
.
15. Patterson C. World Alzheimer report 2018 [Internet] London (UK): Alzheimer’s Disease International; c2018 [cited 2022 May 22]. Available from: https://www.alz.co.uk/research/world-report-2018.AsDIWarF
.
16. Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:88-106.


17. Guoqing Z, Fang S, Lihui D, Bing Y, Qiaoling P, Yingting W, et al. Cerebral white matter lesions and silent cerebral infarcts in postmenopausal women with polycystic ovary syndrome. Gynecol Endocrinol 2016;32:655-8.


18. Harvey RJ, Skelton-Robinson M, Rossor MN. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry 2003;74:1206-9.



19. Hughes TF, Ganguli M. Modifiable midlife risk factors for late-life cognitive impairment and dementia. Curr Psychiatry Rev 2009;5:73-92.



20. Bove RM, Brick DJ, Healy BC, Mancuso SM, Gerweck AV, Bredella MA, et al. Metabolic and endocrine correlates of cognitive function in healthy young women. Obesity (Silver Spring) 2013;21:1343-9.




21. Birdsall MA, Farquhar CM. Polycystic ovaries in pre and post-menopausal women. Clin Endocrinol (Oxf) 1996;44:269-76.



22. Elting MW, Korsen TJ, Rekers-Mombarg LT, Schoemaker J. Women with polycystic ovary syndrome gain regular menstrual cycles when ageing. Hum Reprod 2000;15:24-8.

23. Brown ZA, Louwers YV, Fong SL, Valkenburg O, Birnie E, de Jong FH, et al. The phenotype of polycystic ovary syndrome ameliorates with aging. Fertil Steril 2011;96:1259-65.


24. Noble H, Smith J. Reviewing the literature: choosing a review design. Evid Based Nurs 2018;21:39-41.


25. Collins JA, Fauser BC. Balancing the strengths of systematic and narrative reviews. Hum Reprod Update 2005;11:103-4.


26. Chaney MA. So you want to write a narrative review article? J Cardiothorac Vasc Anesth 2021;35:3045-9.


27. Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev 2019;4:5.




28. Dumitrescu R, Mehedintu C, Briceag I, Purcarea VL, Hudita D. The polycystic ovary syndrome: an update on metabolic and hormonal mechanisms. J Med Life 2015;8:142-5.


29. Walters KA, Gilchrist RB, Ledger WL, Teede HJ, Handelsman DJ, Campbell RE. New perspectives on the pathogenesis of PCOS: neuroendocrine origins. Trends Endocrinol Metab 2018;29:841-52.


30. Upadhayay N, Guragain S. Comparison of cognitive functions between male and female medical students: a pilot study. J Clin Diagn Res 2014;8:BC12-5.



31. Ciocca G, Limoncin E, Carosa E, Di Sante S, Gravina GL, Mollaioli D, et al. Is testosterone a food for the brain? Sex Med Rev 2016;4:15-25.


32. Pintzka CW, Evensmoen HR, Lehn H, Håberg AK. Changes in spatial cognition and brain activity after a single dose of testosterone in healthy women. Behav Brain Res 2016;298:78-90.

33. Rees DA, Udiawar M, Berlot R, Jones DK, O’Sullivan MJ. White matter microstructure and cognitive function in young women with polycystic ovary syndrome. J Clin Endocrinol Metab 2016;101:314-23.



34. McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev 1999;20:279-307.


35. Leeners B, Kruger THC, Geraedts K, Tronci E, Mancini T, Ille F, et al. Lack of associations between female hormone levels and visuospatial working memory, divided attention and cognitive bias across two consecutive menstrual cycles. Front Behav Neurosci 2017;11:120.



36. Sundström Poromaa I, Gingnell M. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci 2014;8:380.



37. Pessoa L. Emotion and cognition and the amygdala: from “what is it?” to “what’s to be done?”. Neuropsychologia 2010;48:3416-29.



38. Barry JA, Parekh HS, Hardiman PJ. Visual-spatial cognition in women with polycystic ovarian syndrome: the role of androgens. Hum Reprod 2013;28:2832-7.


39. Lei Y, Renyuan Z. Effects of androgens on the amyloid-β protein in Alzheimer’s disease. Endocrinology 2018;159:3885-94.


40. Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, Melcher K, et al. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin 2017;38:1205-35.




41. Schattmann L, Sherwin BB. Testosterone levels and cognitive functioning in women with polycystic ovary syndrome and in healthy young women. Horm Behav 2007;51:587-96.


42. Kara O, Kaymaz N, Uzun ME. The effect of hyperandrogenism and obesity on mindfulness and metacognition in adolescents with polycystic ovary syndrome. Arch Womens Ment Health 2022;25:911-21.



43. Lai W, Li X, Zhu H, Zhu X, Tan H, Feng P, et al. Plasma luteinizing hormone level affects the brain activity of patients with polycystic ovary syndrome. Psychoneuroendocrinology 2020;112:104535.


44. Rao CV. Involvement of luteinizing hormone in Alzheimer disease development in elderly women. Reprod Sci 2017;24:355-68.



45. Ali SA, Begum T, Reza F. Hormonal influences on cognitive function. Malays J Med Sci 2018;25:31-41.



46. Jamil AS, Alalaf SK, Al-Tawil NG, Al-Shawaf T. Comparison of clinical and hormonal characteristics among four phenotypes of polycystic ovary syndrome based on the Rotterdam criteria. Arch Gynecol Obstet 2016;293:447-56.



47. Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2017;32:1075-91.


48. Sukhapure M. Androgens and the female brain: the relationship between testosterone levels, depression, anxiety, cognitive function, and emotion processing in females with polycystic ovarian syndrome [Internet] Dunedin (NZ): University of Otago; c2019 [cited 2022 May 1]. Available from: https://ourarchive.otago.ac.nz/bitstream/handle/10523/9347/SukhapureMayouri2018PhD.pdf?sequence=1&isAllowed=y
.
49. Mehrabadi S, Jahanian Sadatmahalleh S, Kazemnejad A. Association of depression and anxiety with cognitive function in patients with polycystic ovary syndrome. J Mazandaran Univ Med Sci 2017;27:159-70.
50. Llewellyn DJ, Lang IA, Langa KM, Huppert FA. Cognitive function and psychological well-being: findings from a population-based cohort. Age Ageing 2008;37:685-9.



51. Marin MF, Lord C, Andrews J, Juster RP, Sindi S, Arsenault-Lapierre G, et al. Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem 2011;96:583-95.


52. Cooney LG, Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Fertil Steril 2018;110:794-809.


53. Kahal H, Kyrou I, Uthman OA, Brown A, Johnson S, Wall PDH, et al. The prevalence of obstructive sleep apnoea in women with polycystic ovary syndrome: a systematic review and meta-analysis. Sleep Breath 2020;24:339-50.




54. Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis 2015;7:920-9.


55. Krysta K, Bratek A, Zawada K, Stepańczak R. Cognitive deficits in adults with obstructive sleep apnea compared to children and adolescents. J Neural Transm (Vienna) 2017;124:187-201.




56. Karapin P, Šiarnik P, Suchá B, Jurík M, Tedla M, Poddaný M, et al. Cognition in patients with sleep-disordered breathing: can obstructive and central apneic pauses play a different role in cognitive impairment? Life (Basel) 2022;12:1180.



57. Lajoie AC, Lafontaine AL, Kimoff RJ, Kaminska M. Obstructive sleep apnea in neurodegenerative disorders: current evidence in support of benefit from sleep apnea treatment. J Clin Med 2020;9:297.



58. Harrington YA, Parisi JM, Duan D, Rojo-Wissar DM, Holingue C, Spira AP. Sex hormones, sleep, and memory: interrelationships across the adult female lifespan. Front Aging Neurosci 2022;14:800278.



59. Moura HH, Costa DL, Bagatin E, Sodré CT, Manela-Azulay M. Polycystic ovary syndrome: a dermatologic approach. An Bras Dermatol 2011;86:111-9.


60. Deveci E, Öztürk A, Kırpınar I, Koyuncu A, Engin I, Melikoğlu M, et al. Neurocognition in patients with acne vulgaris. J Psychiatry 2014 Apr 30 [Epub]. https://doi.org/10.4172/Psychiatry.1000121
.

61. Mehrabadi S, Jahanian Sadatmahalleh SH, Kazemnejad A. The relationship between acne and cognitive function in patients with polycystic ovary syndrome and healthy women. J Guilan Univ of Med Sci 2018;27:37-42.
62. Golchai J, Khani SH, Heidarzadeh A, Eshkevari SS, Alizade N, Eftekhari H. Comparison of anxiety and depression in patients with acne vulgaris and healthy individuals. Indian J Dermatol 2010;55:352-4.



63. Yazici K, Baz K, Yazici A, Köktürk A, Tot S, Demirseren D, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol 2004;18:435-9.


64. Perini G, Cotta Ramusino M, Sinforiani E, Bernini S, Petrachi R, Costa A. Cognitive impairment in depression: recent advances and novel treatments. Neuropsychiatr Dis Treat 2019;15:1249-58.


65. Jafferany M, Patel A. Understanding psychocutaneous disease: psychosocial & psychoneuroimmunologic perspectives. Int J Dermatol 2020;59:8-15.



66. Mashayekhi Goyonlo V, Sardabi MS, Tavalaei AM, Khoshnevisan Z, Razmara M. Cognitive behavioral therapy as an adjuvant therapy in acne excoriée: a randomized controlled clinical trial. J Dermatolog Treat 2022;33:782-8.


67. Tavares A, Rêgo Barros RC. The prevalence of metabolic syndrome in the different phenotypes of polycystic ovarian syndrome. Rev Bras Ginecol Obstet 2019;41:37-43.



68. Pillai BP, Kumar H, Jayakumar RV, Alur V, Sheejamol VS. The prevalence of metabolic syndrome in polycystic ovary syndrome in a South Indian population and the use of neck circumference in defining metabolic syndrome. Int J Diabetes Dev Ctries 2015;35:469-75.


69. Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol 2012;32:2060-7.


70. Lujan ME, Mergler R. Cognitive function in women with polycystic ovary syndrome (PCOS): impact of reproductive and metabolic factors. Fertil Steril 2015;104:E129.

71. Neergaard JS, Dragsbæk K, Christiansen C, Nielsen HB, Brix S, Karsdal MA, et al. Metabolic syndrome, insulin resistance, and cognitive dysfunction: does your metabolic profile affect your brain? Diabetes 2017;66:1957-63.



72. van Gemert T, Wölwer W, Weber KS, Hoyer A, Strassburger K, Bohnau NT, et al. Cognitive function is impaired in patients with recently diagnosed type 2 diabetes, but not type 1 diabetes. J Diabetes Res 2018;2018:1470476.


73. Castellano CA, Baillargeon JP, Nugent S, Tremblay S, Fortier M, Imbeault H, et al. Regional brain glucose hypometabolism in young women with polycystic ovary syndrome: possible link to mild insulin resistance. PLoS One 2015;10:e0144116.



74. Jarrett BY, Vantman N, Mergler RJ, Brooks ED, Pierson RA, Chizen DR, et al. Dysglycemia, not altered sex steroid hormones, affects cognitive function in polycystic ovary syndrome. J Endocr Soc 2019;3:1858-68.




75. Zheng F, Yan L, Yang Z, Zhong B, Xie W. HbA1c, diabetes and cognitive decline: the English Longitudinal Study of Ageing. Diabetologia 2018;61:839-48.




76. Janssen J, van den Berg E, Zinman B, Espeland MA, Geijselaers SLC, Mattheus M, et al. HbA1c, insulin resistance, and β-cell function in relation to cognitive function in type 2 diabetes: the CAROLINA cognition substudy. Diabetes Care 2019;42:e1-3.




77. Huang Y, Wang A. Cognitive disorder in women with polycystic ovary syndrome: a remote complication. J Pediatr Adolesc Gynecol 2014;27:240-1.


78. Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2012;18:618-37.


79. Cook RL, O’Dwyer NJ, Donges CE, Parker HM, Cheng HL, Steinbeck KS, et al. Relationship between obesity and cognitive function in young women: the food, mood and mind study. J Obes 2017;2017:5923862.




80. Hou Q, Guan Y, Yu W, Liu X, Wu L, Xiao M, et al. Associations between obesity and cognitive impairment in the Chinese elderly: an observational study. Clin Interv Aging 2019;14:367-73.


81. Nguyen JC, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci 2014;8:375.



82. Li G, Hu J, Zhang S, Fan W, Wen L, Wang G, et al. Changes in resting-state cerebral activity in women with polycystic ovary syndrome: a functional mr imaging study. Front Endocrinol (Lausanne) 2020;11:603279.



83. Mehrabadi S, Sadatmahalleh SJ, Kazemnejad A, Moini A. Association of acne, hirsutism, androgen, anxiety, and depression on cognitive performance in polycystic ovary syndrome: a cross-sectional study. Int J Reprod Biomed 2020;18:1049-58.




84. Sukhapure M, Eggleston K, Douglas K, Fenton A, Frampton C, Porter RJ. Free testosterone is related to aspects of cognitive function in women with and without polycystic ovary syndrome. Arch Womens Ment Health 2022;25:87-94.



85. Shi Y, Cui Y, Sun X, Ma G, Ma Z, Gao Q, et al. Hypertension in women with polycystic ovary syndrome: prevalence and associated cardiovascular risk factors. Eur J Obstet Gynecol Reprod Biol 2014;173:66-70.


86. Joham AE, Boyle JA, Zoungas S, Teede HJ. Hypertension in reproductive-aged women with polycystic ovary syndrome and association with obesity. Am J Hypertens 2015;28:847-51.


87. Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension 2016;68:e67-94.



89. Read SL, Grundy EMD. Fertility history and cognition in later life. J Gerontol B Psychol Sci Soc Sci 2017;72:1021-31.



90. Saenz JL, Díaz-Venegas C, Crimmins EM. Fertility history and cognitive function in late life: the case of Mexico. J Gerontol B Psychol Sci Soc Sci 2021;76:e140-52.




91. Palomba S, Santagni S, Falbo A, La Sala GB. Complications and challenges associated with polycystic ovary syndrome: current perspectives. Int J Womens Health 2015;7:745-63.



92. Karim R, Dang H, Henderson VW, Hodis HN, StJohn J, Brinton RD, et al. Effect of reproductive history and exogenous hormone use on cognitive function in mid- and late life. J Am Geriatr Soc 2016;64:2448-56.




93. Prince MJ, Acosta D, Guerra M, Huang Y, Jimenez-Velazquez IZ, Llibre Rodriguez JJ, et al. Reproductive period, endogenous estrogen exposure and dementia incidence among women in Latin America and China; a 10/66 population-based cohort study. PLoS One 2018;13:e0192889.



94. Peterson A, Tom SE. A lifecourse perspective on female sex-specific risk factors for later life cognition. Curr Neurol Neurosci Rep 2021;21:46.




95. Xu XL, Deng SL, Lian ZX, Yu K. Estrogen receptors in polycystic ovary syndrome. Cells 2021;10:459.



96. Schelbaum E, Loughlin L, Jett S, Zhang C, Jang G, Malviya N, et al. Association of reproductive history with brain MRI biomarkers of dementia risk in midlife. Neurology 2021;97:e2328-39.



97. Leeners B, Krüger T, Geraedts K, Tronci E, Mancini T, Ille F, et al. Cognitive function in association with high estradiol levels resulting from fertility treatment. Horm Behav 2021;130:104951.


98. Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565-92.



99. Warren AM, Gurvich C, Worsley R, Kulkarni J. A systematic review of the impact of oral contraceptives on cognition. Contraception 2014;90:111-6.


100. Botsali A, Kocyigit P, Uran P. The effects of isotretinoin on affective and cognitive functions are disparate in adolescent acne vulgaris patients. J Dermatolog Treat 2020;31:734-8.


101. Soleman RS, Kreukels BPC, Veltman DJ, Cohen-Kettenis PT, Hompes PGA, Drent ML, et al. Does polycystic ovary syndrome affect cognition? A functional magnetic resonance imaging study exploring working memory. Fertil Steril 2016;105:1314-21e1.


102. Schattmann L, Sherwin BB. Effects of the pharmacologic manipulation of testosterone on cognitive functioning in women with polycystic ovary syndrome: a randomized, placebo-controlled treatment study. Horm Behav 2007;51:579-86.


103. Alzaid A, Tourkmani A, Alsahaby A, Robert A, Bin Rsheed A, Alabood A, et al. Metformin therapy improves cognitive function in women with polycystic ovary syndrome (PCOS). Diabetologia 2017;60(Suppl 1):581-2.

104. Liu J, Wu Q, Hao Y, Jiao M, Wang X, Jiang S, et al. Measuring the global disease burden of polycystic ovary syndrome in 194 countries: Global Burden of Disease Study 2017. Hum Reprod 2021;36:1108-19.




105. Park CH, Chun S. Influence of combined oral contraceptives on polycystic ovarian morphology-related parameters in Korean women with polycystic ovary syndrome. Obstet Gynecol Sci 2020;63:80-6.




106. Moradi N, Bidgoli SA, Chaichian S. Ovarian cysts disappear after 14-day oral regimen of Korean red ginseng extract in letrozole-induced polycystic ovarian syndrome. Obstet Gynecol Sci 2021;64:874-83.




107. Pasquali R. Contemporary approaches to the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab 2018;9:123-34.




108. Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab 2020;35:100937.



109. Van Duinkerken E, Snoek FJ, de Wit M. The cognitive and psychological effects of living with type 1 diabetes: a narrative review. Diabet Med 2020;37:555-63.




110. Sutin AR, Stephan Y, Terracciano A. Psychological distress, self-beliefs, and risk of cognitive impairment and dementia. J Alzheimers Dis 2018;65:1041-50.



111. Guicciardi M, Crisafulli A, Doneddu A, Fadda D, Lecis R. Effects of metabolic syndrome on cognitive performance of adults during exercise. Front Psychol 2019;10:1845.



112. Kim B, Feldman EL. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp Mol Med 2015;47:e149.




113. Ozgen Saydam B, Yildiz BO. Polycystic ovary syndrome and brain: an update on structural and functional studies. J Clin Endocrinol Metab 2020;106:e430-41.


114. Gaspar JM, Baptista FI, Macedo MP, Ambrósio AF. Inside the diabetic brain: role of different players involved in cognitive decline. ACS Chem Neurosci 2016;7:131-42.


115. Rocha AL, Oliveira FR, Azevedo RC, Silva VA, Peres TM, Candido AL, et al. Recent advances in the understanding and management of polycystic ovary syndrome. F1000Res 2019 Apr 26 [Epub]. https://doi.org/10.12688/f1000research.15318.1
.

116. Boivin MJ, Fatehi F, Phillips-Chan AE, Richardson JR, Summers AN, Foley SA. Exploratory study of a screening measure for polycystic ovarian syndrome, quality of life assessment, and neuropsychological evaluation. BMC Womens Health 2020;20:132.




117. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018;33:1602-18.




118. Barry JA. Psychological aspects of polycystic ovary syndrome. London (UK): Palgrave Macmillan; 2019.
119. Ranjbar F, Moazeni F, Abdi M, Khoshnou H, Khademi E, Amiri S, et al. Evaluation of spatial memory in women with polycystic ovary syndrome. Iran J Obstet Gynecol Infertil 2020;23:1-9.
- TOOLS
-
METRICS

- Related articles in Obstet Gynecol Sci
-
Delta neutrophil index in obese and non-obese polycystic ovary syndrome patients2023 September;66(5)
Selective oocyte retrieval in patients with polycystic ovarian disease.1992 July;35(7)