The introduction to air pollution on pregnancy outcome (APPO) study: a multicenter cohort study
Article information
Abstract
Objective
The air pollution on pregnancy outcome (APPO) study is a prospective hospital-based cohort study designed to investigate the maternal and fetal effects of a particulate matter with an aerodynamic below 10 μm (PM10) and PM2.5 (below 2.5 μm) exposure. This study aims to analyze a relationship between particulate matter and adverse pregnancy outcomes and to find related biomarkers and develop management guidelines.
Methods
About 1,200 pregnant women are recruited for 3 years (from January 2021 to December 2023) from seven university hospitals to investigate the effects of particulate matter on pregnancy complications and adverse pregnancy outcomes. We collect biological samples by 5 mL of maternal venous blood and 15 mL of urine in each trimester of pregnancy, and 5 mL of umbilical cord blood and 2×2×2 cm of placental tissue are collected after delivery. In addition, by applying PM10 and PM2.5 concentration values and time-activity patterns from the time weighted average model, the individual predicted exposure of air pollution for the pregnant women are obtained.
Results
The average exposure of PM10 and PM2.5 of the participants in the entire period of pregnancy, was exceeded the World Health Organization air quality guidelines (an annual level, PM10 >15 μg/m3, PM2.5 >5 μg/m3). Moreover, it was revealed that the PM concentration was increasing toward the 3rd trimester of pregnancy.
Conclusion
The APPO study will be able to identify the degree of exposure to air pollution in pregnant women and use it as basic data for estimating individual exposure to particulate matter. And the results of the APPO study will facilitate in the development of health management for pregnant women against air pollution.
Introduction
Air pollution, a mixture of pollutants found in the air, is a major environmental problem in public health. In particular, the effect of perinatal exposure to air pollution is a significant concern because pregnant women are more vulnerable to environmental toxicants [1–4]. Many studies have shown an association between maternal exposure to air pollution and adverse pregnancy outcomes [2,5–13]. These studies suggest that maternal exposure to air pollution may increase the risk of adverse birth outcomes, including abortion, stillbirth, preterm birth (PTB), low birth weight (LBW), intrauterine growth retardation, and neonatal mortality [13–15]. Although particulate matter with an aerodynamic diameter <10 μm (PM10) has been on the decline since 2002 in Korea, the average annual PM10 and PM2.5 (particulate matter with an aerodynamic below 2.5 μm) concentrations in Seoul are still higher than those in major cities in developed countries [16]. Particularly, PM2.5, which has been measured in Korea since 2015, is very small and not filtered through skin pores and bronchial tubes; therefore, it penetrates blood vessels and placenta, causing an inflammatory reaction [16–18]. In addition, the airborne spread of severe acute respiratory syndrome coronavirus 2 virus (coronavirus disease 2019, [COVID-19]), which was outbroken from the end of 2019, has more adverse effects on human respiratory health, and a positive correlation has been observed between its viral spread and air pollution [19,20]. Atmospheric PM could create a suitable environment for transporting the virus to greater distances and induce inflammation in lung cells, thereby increasing the susceptibility of patients and severity of the disease course in COVID-19 [19,20]. These processes can be more lethal because pregnant women are vulnerable to respiratory system [21,22].
Previous studies have reported that adverse pregnancy outcomes such as LBW and PTB are related to air pollution using regional ambient PM10 data from the residence of pregnant women and national birth data [12,15,23]. However, these studies are limited in that they do not reflect the exposure level of modern people in the real world who mainly live indoors and, therefore, have to deal with indoor air quality, rather than outdoor air quality. In addition, the studies could not analyze in detail exposure status according to each individual’s lifestyle.
The air pollution on pregnancy outcome (APPO) study aims to analyze adverse pregnancy outcomes, related to particulate matter, identify related biomarkers, and develop management guidelines. We will establish individual air pollution exposure methods and collect and analyze integrated information on the effects of each exposure level on pregnancy outcomes. In addition, we will aim to discover predictive factors for pregnancy complications caused by PM10 and PM2.5 and develop management indicators for pregnant women. Thus, this article introduces the planning processes, study design, protocols, current status, and future directions of the APPO study.
Materials and methods
1. APPO study design
1) Basic concept and process
The APPO study is a prospective hospital-based cohort study designed to investigate the effects of PM10 and PM2.5 exposure on mothers and fetuses. Pregnant women were included in this study. To develop the specific hypotheses relevant to PM exposure and the maternal and fetal effects, our team reviewed a list of hypotheses from previous studies, including published articles, reports, and textbooks. Based on this review, key research hypotheses were established, and medical, social, and other factors that can affect pregnant women and fetuses were considered. Thus, we generated key hypotheses regarding pregnancy, childbirth, and neonatal prognosis associated with environmental exposure (Table 1).
2) Cohort composition
The APPO study plans to investigate the effects of particulate matter on mothers and fetuses by recruiting more than 1,200 participants from January 2021 to December 2023. Six university hospitals (Kangwon National University Hospital, Keimyung University Dongsan Medical Center, Korea University Guro Hospital, Severance Hospital, Ewha Womans University Mokdong Hospital, and Ewha Womans University Seoul Hospital) participated in 2021, and Ulsan University Hospital was included in 2022. The hospitals were located in a metropolitan area (Seoul), an industrial complex (Guro-gu in Seoul, Ulsan), and a mountainous area (Gangwon-do) to fully reflect the characteristics of different regions. Seoul was selected as an important area for this study because of its large population, high traffic volume, severe air pollution, and several apartments [24]. Moreover, Ulsan was selected as another research area as the largest industrial city in South Korea [24].
2. Study protocol
1) Recruitment of participants
To investigate the effects of particulate matter on pregnancy complications and adverse pregnancy outcomes, early pregnant women without underlying diseases were selected for the study. The inclusion and exclusion criteria are presented in Table 2. Participants were excluded if they withdrew their consent or could not provide information on delivery because of follow-up loss. When the participants visited the hospital for regular evaluation in the 1st, 2nd, and 3rd trimester of pregnancy, we collected information related to pregnancy complications through questionnaires, ultrasound sonography, and blood and urine tests. After delivery, umbilical cord blood and placental tissue were collected, and information regarding pregnancy outcomes was obtained from medical records. A flowchart of the APPO study is shown in Fig. 1.
2) Collection of biological samples
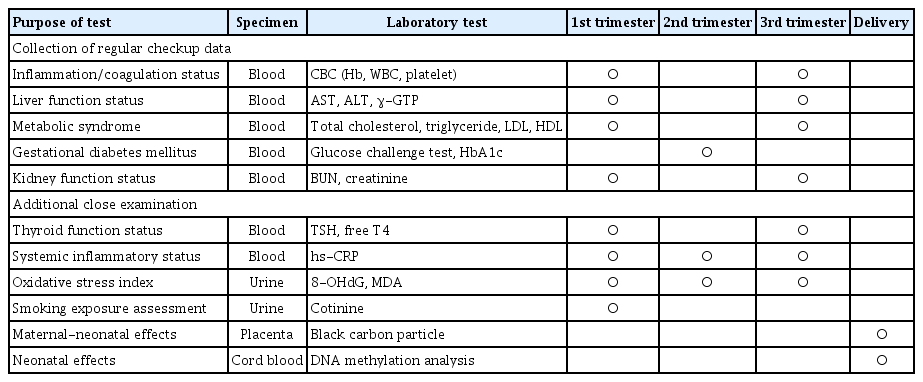
Biological samples collected include 5 mL of maternal venous blood and 15 mL of urine in each trimester of pregnancy; in addition, 5 mL of umbilical cord blood and 2×2×2 cm placental tissue were collected after delivery. Placental tissue was mainly collected at the Ewha Womans University Mokdong Hospital, a representative institute. Biological samples were collected when participants visited for regular evaluation to avoid discomfort, and all biological samples were collected after obtaining informed consent from the participants. Whole blood samples collected in ethylene-diamine-tetraacetic acid tubes were dispensed into cryo-tubes (1.0 mL), and urine was stored in a cryo-tube (10 mL). Samples for long-term storage were transferred to the institution (Seegene Medical Foundation, Seoul, Korea) on the same day by refrigeration to prevent deterioration. The storage samples were labeled with a unique number, collection date, and sample name; afterward, they were systematically stored and managed in a laboratory freezer (−80°C) after being delivered by the Department of Obstetrics and Gynecology at Ewha Womans University Mokdong Hospital. Specific laboratory test results for each biological specimen are presented in Table 3. Tests for systemic diseases, such as inflammation, thyroid disease, and metabolic syndrome, were mainly analyzed from blood samples, and additional tests, such as oxidative stress, were analyzed from urine samples. 8-hydroxy-2′-deoxyguanosine (8-OHdG) is an oxidized nucleoside of DNA frequently detected in damaged nuclear and mitochondrial DNA; and 8-OHdG is excreted in the urine following DNA repair [25]. There is much evidence that malondialdehyde (MDA) is not only a biomarker of generalized cellular oxidative stress, but may also be a risk factor for specific diseases [25]. MDA is the most studied product of polyunsaturated fatty acid peroxidation and is related to lipid oxidation among oxidative stress markers [26]. Moreover, the placenta and umbilical cord blood are mainly used to test the effects in neonates. Black carbon is a major component of the fine dust produced during the combustion of fossil fuels and biomass. It is small enough to be inhaled and deposited directly in the lungs [27]. Therefore, we hypothesized that it can eventually be deposited in the placenta through a blood vessel and observable in the placental tissue. Furthermore, DNA methylation is an epigenetic mechanism related to the fetal programming theory, which states that the environment in utero affects fetal gene expression. Therefore, this study plans to analyze the DNA methylation status in umblical cord blood to predict its effect on the fetus (Table 3).
3) Assessment of outcomes: clinical information
We collected the following clinical information from each mother’s medical record: prenatal clinical information (cervical length, blood pressure), labor and delivery (delivery date, gestational age, mode of delivery, weight of placenta), pregnancy complications (preterm premature rupture of membrane, dystocia, cephalopelvic disproportion, breech or other abnormal presentation, placenta previa, placental abruption, obstetrical hemorrhage, precipitating labor, PTB, umbilical cord prolapse, eclampsia), neonatal data (sex, height, weight, 1′ and 5′ Apgar score), neonatal complications (meconium aspiration syndrome, fetal asphyxia), and other information (fever, anesthetic use). In addition, oxidative stress index, smoking exposure, carbon particle analysis, and analysis of cord blood DNA methylation are required for risk analysis of particulate matter; therefore, they would be analyzed in high PM10 and PM2.5 concentrations.
4) Data control and management
We used clinical research and trial management system (iCReaT ver. 2, National Institute of Health, Cheongju, Korea) a web-based system, to input, store, and integrate the aforementioned data. iCReaT was developed to provide evidence-based health care services in an efficient and integrated manner by the National Institute of Health in Korea; it oversees clinical research, including clinical trials, research plan review, project management, data input and extraction, and report provision [28]. Data quality was monitored periodically, the program automatically screened, and additional investigations conducted to obtain information about the questionnaire items left blank or insufficient answers were supplemented. The iCReaT system was suitably configured to handle the special requirements of the APPO study, and a training program for its use was provided for all administrators and researchers. Access to all data was limited to the certified researchers in the study protocol.
3. Calculation of estimated individual exposure to PM
1) Questionnaires
In this study, we investigated the current status of PM that pregnant women in Korea are exposed to indoors through a web-based questionnaire (kopen.or.kr), and we collected information on the participants’ sociodemographic characteristics, lifestyle, living environment, and risk perception level of fine dust. This questionnaire was developed by a cohort of housewives, and it was possible to indirectly evaluate exposure to air pollution by examining house occupancy, cooking, and cleaning times, which are factors that generate indoor fine dust, and indoor/outdoor ventilation (number of windows opened). These data were input and stored in the iCReaT system of each institution, and a representative research director integrated and managed the data.
In addition, it was recommended that all participants fill out a time-activity log on the web-based questionnaire. They recorded the information using a time-activity diary, and activity information was collected at 1-hour intervals. The activities were organized according to the following classifications: main activity, extra activity, transportation, indoors, and outdoors. Participants’ time activities were analyzed to identify the indoor and outdoor residence time patterns. The identified pattern was used to calculate individual exposure to indoor and outdoor PM10 and PM2.5.
2) Measurement of outdoor air pollution concentration
The outdoor PM concentrations (PM10 and PM2.5) were collected from a nearby urban atmospheric measurement network based on the addresses of the pregnant women who participated in the study. The urban air monitoring station data used in this study were obtained from air Korea (https://www.airkorea.or.kr/web), which is operated by the Korean Ministry of Environment. Air Korea has been disclosing its national real-time air pollution level information via a website since December 2005 [29]. As at 2021, there were 614 stations in 162 cities and counties in Korea for air quality monitoring of sulfur dioxide, nitrogen dioxide, ozone, carbon monoxide, PM10, and PM2.5 [29].
3) Measurement of indoor air pollution concentration
The indoor concentrations of PM10 and PM2.5 were measured by placing a fine dust meter in the living room of each participant’s home. AirGuardK (Kweather Co, Seoul, Korea) was used to measure fine particles (PM10, PM2.5), carbon dioxide (CO2), volatile organic compounds (VOCs), temperature, humidity, and noise. These measurements were taken online at 1-minute intervals. The measured indoor air quality data are stored in the indoor air quality monitoring platform through the long-term evolution communication network to prevent loss. In addition, the data are collected and stored every minute. Measurements were carried out for a week during each trimester of pregnancy, and the measured concentration value was determined in real time using the internet of things and information and communication technology.
4) Personal exposure prediction using a time-weighted average model
We used a time-weighted average model to estimate the individual PM exposure in pregnant women. The time-weighted average model is a concept in which an individual’s exposure over a 24-hour period can be expressed as the sum of exposure to each microenvironment weighted to a time-activity pattern. Although it was not possible to measure the concentration of air pollutants in all local environments, individual exposure to PM10 and PM2.5, using a time log and time-weighted model, can be predicted [25–28]. The time-weighted model can be expressed by the following equations: Cper=∑(Tindoor×Cindoor+Toutdoor×Coutdoor) where, Cper: predicted personal exposure, Tindoor: indoor activity time rate (indoor activity time/24hr), Cindoor: indoor PM2.5 concentration, Toutdoor: outdoor activity time rate (outdoor activity time/24hr). Coutdoor: outdoor PM2.5 concentration.
In this study, by applying PM10 and PM2.5 concentration values and temporal activity patterns from the time-weighted average model, predicted individual exposure values for pregnant women was obtained. For pregnant women who worked in the office, PM10 and PM2.5 concentrations at the workplace were excluded because of the lack of measurement data. As a result, a time-weighted average model of indoor and outdoor PM10 and PM2.5 concentration values and temporal activity patterns was applied to represent the predicted values for individual exposure.
Results
1. General characteristics of participants
Table 4 shows the distribution of sociodemographic characteristics, living environment, lifestyle, and clinical details for 333 participants of the APPO study by June 2022 who had completed childbirth. The mean age of the pregnant women was 33.6 years, and their pre-pregnancy body mass index was 22.2, which was within the normal range. Most participants were married college graduates. They were unlikely to smoke or drink alcohol during pregnancy; however, 47.1% consumed coffee during pregnancy. Additionally, most participants lived in urban areas (92.8%), 3.6% in rural and mountainous areas, and 0.6% in industrial complexes. They were naturally pregnant and delivered at an average of 38 weeks and 4 days of gestation. The proportions of male (n=179, 53.8%) and female (n=152, 45.6%) neonates were similar, and the mean birth weight was 3,138 g. The incidence of PTB was 7.8% and 2.7% of the newborns had LBW (Table 4).
2. Individual exposure to PM10 and PM2.5 concentrations
The estimated individual exposure amounts of PM10 and PM2.5 for participants are shown in Table 5. PM10 and PM2.5 exposures were presented as values for each trimester of pregnancy and the entire period of pregnancy. The average exposure of PM10 and PM2.5 among the participants in the entire period of pregnancy exceeded the World Health Organization air quality guidelines (an annual level, PM10 >15 μg/m3, PM2.5 >5 μg/m3) [30]. Moreover, it was revealed that the PM concentration was increasing toward the 3rd trimester of pregnancy. Based on these results, it is possible to analyze the relationship between PM and pregnancy outcomes.
Discussion
The APPO study aims to analyze adverse pregnancy outcomes resulting from PM exposure, identify related biomarkers, and develop management guidelines. In detail, about 1,200 pregnant women will be recruited over 3 years to collect data on fine dust exposure; in addition, methods for measuring exposure to fine particles for each pregnant woman and estimating output will be established. Standardization of the protocol used in this APPO study will play an important role in establishing the study design and its future applications. Moreover, it could provide a basis for estimating individual exposure to PM and identifying air pollution sources. Furthermore, it could influence the development of action points and policy support directions for disease control in pregnant women.
The first strength of this study is the direct collection of measured data on indoor fine dust considering the lifestyle of pregnant women. Periodic and repeated measures of environmental exposure during pregnancy could provide a unique and powerful opportunity for etiological investigation of various environmental exposures at different time points and their effects on maternal health. In addition, the concentration of outdoor PM and individual exposure estimates were calculated using a time-activity log to reflect the actual exposure level as accurately as possible. Second, this study had a sufficiently large sample size to investigate maternal and fetal health outcomes. Participants were recruited from various areas in Korea to ensure that the study results are representative of all parts of the country. Furthermore, the variability in environmental exposure data for each area provides a good opportunity to detect the environmental impacts on health outcomes. Finally, the influence of multiple environmental exposures on various health outcomes was assessed. Air pollutants such as PM10, PM2.5, CO2, VOCs, temperature, humidity, and noise were directly measured indoors. Thus, the results could be analyzed for synergistic effects, beyond the effect on a single substance, when multiple environmental pollutants were exposed simultaneously. This APPO study will elucidate the mechanisms involved and expand relevant research on the potential interactions among several risk factors.
However, the study had some limitations that need to be addressed. It was impossible to measure all possible environmental exposures, despite the development of extensive measurement techniques and individual activity analyses. In addition, all specific organs or bodily fluids could not be collected for genomic studies, despite urine, blood, and umbilical cord blood samples being collected to analyze exposure detection and identification of predictive biomarkers.
Recently, air pollution and abnormal climate change have become serious and considerable issues worldwide. In addition, securing the health of pregnant women and fetuses is necessary because of the low fertility rate and increase in the proportion of high-risk pregnancies in Korea. Notably, continuous attention to maternal environment and health is needed for a sustainable society. Therefore, we conducted a prospective cohort study on pregnant women. We expect that this APPO study will identify the degree of exposure to air pollution among pregnant women and use the results as basic data for estimating individual exposure to fine dust. The results of this APPO study will facilitate the development of health management strategies for pregnant women against air pollution.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study protocol was reviewed and approved by the Institutional Review Board of the Ewha Womans University College of Medicine (approval no. 2021-04-032). Informed consent was obtained from all the participants when they were enrolled for the study.
Patient consent
Written informed consent was obtained from all participants before their inclusion in the study.
Funding information
This research was supported by the “National Institute of Health” Research Project (project no. #2021-ER1208-01).