Intraumbilical versus intramuscular oxytocin in the management of the third stage of labor
Article information
Abstract
Objective
To compare the effect of intraumbilical vein oxytocin injection with standard management in reducing blood loss during the third and fourth stages of labor. Acute complications threaten the mother’s life during the third and fourth stages of labor. The most common complication is postpartum hemorrhage, which remains a leading cause of maternal mortality, particularly in developing countries.
Methods
A randomized controlled trial was conducted in the Gynecology and Obstetrics Department of Leonardo Martínez Valenzuela Hospital from January to June 2021. A probabilistic sample was used: 332 pregnant patients were enrolled in the study and randomized into the case (166 patients) and control (166 patients) groups. The volume of blood lost was compared between the groups.
Results
The median estimated blood loss was 120 mL (interquartile range [IQR], 80–218.75 mL). There was a statistically significant difference between the groups, showing less estimated blood loss in the international unit group with a median of 80 mL (IQR, 60–100 mL) (P<0.001), and 200 mL (IQR, 143.75–300 mL) in the intramuscular (IM) group, highlighting that 66.8% of the IM group had an estimated blood loss >251 mL.
Conclusion
Any reduction in bleeding during labor is clinically relevant because it improves patient prognosis. The use of intraumbilical oxytocin injection with active management of the third stage of labor significantly reduced postpartum blood loss and the duration of the third stage compared with the IM group.
Introduction
Acute complications threaten the mother’s life during the third and fourth stages of labor. The most common complication is postpartum hemorrhage (PPH), which remains a leading cause of maternal mortality, especially in developing countries [1]. The American College of Obstetricians and Gynecologists’ reVITALize program, to standardize clinical definitions in obstetrics, defines PPH as blood loss ≥1,000 mL or blood loss with signs or symptoms of hypovolemia within 24 hours of delivery, whether a cesarean section or vaginal birth [2].
Overall, hemorrhage accounts for 27.1% of all maternal mortality worldwide [3]. According to the World Health Organization (WHO), in 2015, 20% of maternal deaths in America were a consequence of PPH during or immediately after birth. In addition, it is estimated that 8.2% of women giving birth in Latin America will experience severe PPH requiring transfusion [3]. Risk factors for PPH may be antenatal or intrapartum. PPH can also occur in women with no identifiable risk factors; therefore, clinicians should be prepared to manage this complication at every delivery with anticipation [4].
In the third stage of labor, myometrial contraction is responsible for the separation of placental membranes and hemostasis, achieved by uterine blood vessel constriction as the myometrium contracts [5]. However, when an inadequate contraction of the uterine myometrial cells in response to endogenous oxytocin release occurs, it is known as uterine atony [6]. Uterine atony accounts for 80% of all causes of PPH [4]. A well-recognized initial reflex intervention in PPH involves the stimulation of uterine contractions. Therefore, active management of the third stage of labor, which includes prophylactic injection of 10 international unit (IU) of oxytocin within 2 minutes of birth, is recommended by the WHO for PPH prevention [1].
Oxytocin is a nonapeptide hormone that stimulates the upper uterine segment to contract rhythmically [7]. Owing to its rapid onset of action and safety, it is the first-line uterotonic for this purpose in most settings. Intramuscular (IM) or intravenous oxytocin administration reduces postpartum bleeding by stimulating uterine contractions. Nevertheless, the administration of oxytocin through the umbilical vein allows it to reach the placental bed and uterine wall rapidly, resulting in earlier uterine contraction and placental separation at high rates, decreasing the placental attachment site. The resulting pressure causes the decidua to separate, contributing to the formation of the retroplacental hematoma and accelerating the separation process [8,9]. This technique can decrease the risk of PPH and posterior complications due to bleeding.
Few studies in Latin America have demonstrated the advantages and outcomes of umbilical vein oxytocin administration. This study aimed to estimate the effect of intraumbilical vein oxytocin injection compared with standard management in reducing blood loss during the third and fourth stages of labor and the length of the third and fourth stages.
Materials and methods
A randomized controlled trial was conducted from January to June 2021 in the Gynecology and Obstetrics Department of the Leonardo Martínez Valenzuela Hospital, a second-level care hospital that serves as a major referral center for obstetric care in the entire northwestern area of Honduras, with an average of 18,000 deliveries per year. Patients with no predisposition to PPH, gestational age between 37–42 weeks, cephalic presentation, maternal age between 18–46 years, singleton pregnancy, and vaginal delivery were included. Patients with blood pressure >140/90 mmHg, placenta previa, abnormal placentation (accreta, percreta, or increta), oligohydramnios and anhydramnios, coagulopathies and history of anticoagulant drugs, utero-anatomical abnormalities, history of bleeding in the third trimester, previous cesarean section or gynecological surgery, and a prolonged first stage of labor (14 hours multiparous and 20 hours nulliparous) were excluded.
A probabilistic sample was used; 332 pregnant patients were enrolled in the study and randomized into the case (166 patients) and control (166 patients) groups, who received an intraumbilical oxytocin injection and a standard IM oxytocin injection, respectively. The randomization was systematic; a physician in the labor and delivery room assigned a random number to the patient as admitted due to active labor, using a consecutive order and alternating between cases and controls. The medical personnel who attended the delivery were trained to measure blood loss and control each parameter to be evaluated. All deliveries were attended by the same person, and a specialist in gynecology and obstetrics was present to supervise each delivery in case of any eventuality. The recruited pregnant women were blinded to the group assignment. The midwife and data analysts were not blinded to the group coding, but a protocol was followed to minimize selection bias.
In the study arms, after childbirth, clamping, and sectioning of the umbilical cord above the placental cord clamp, approximately 15 cm from the vagina, 20 IU of oxytocin previously diluted in 18 mL of 0.9% saline solution was injected into the umbilical vein with a 20 mL syringe. Signs of detachment were awaited, and controlled traction of the umbilical cord was performed using the Brandt-Andrews maneuver. In the control group, after delivery, clamping, and sectioning of the umbilical cord, only 10 IU of oxytocin was injected intramuscularly in the patient’s right thigh; signs of detachment were awaited, and the placenta was removed.
In both groups, the time taken for placental separation was measured with a stopwatch, starting from when the fetus emerged until the placenta was completely expelled. The volume of blood collected during the third and fourth periods of labor was measured in a graduated glass cylinder in milliliters (avoiding the mixture of blood with amniotic fluid); the bleeding contained in the absorbent materials such as gauze and compresses used during labor and puerperium was quantified as follows: a compress has a maximum absorption capacity of 100 mL, and a simple gauze has a maximum capacity of 5 mL, which are the main indicators under study. Vital signs and uterine tone were monitored every 15 minutes within the first 2 hours after delivery. During the patient’s immediate postpartum period, 6 hours after delivery, a blood sample was taken for hemoglobin and hematocrit control and compared with the antepartum result taken at the patient’s admission to the labor and delivery room.
Biological variables and the patient’s gynecological-obstetric history were studied: maternal age, parity, abortions; obstetric history variables: previous twin delivery, previous instrumented delivery, previous retained placenta, previous history of manual removal of placenta; delivery variables: type of delivery (eutocic: delivery developed without complications/dystocic: difficult or obstructed delivery), method of placental separation (Schultze/Matthew Duncan), completeness of placental membranes (complete/incomplete), and PPH (blood loss >500 mL or >1,000 mL is considered an indicator of PPH). Other variables during the third stage of labor were considered: remaining undelivered placentas, retained placenta, manual placenta removal, changes in hematocrit concentration, and control hemoglobin.
At the institutional level, as a protocol regulation, active management of the third stage of labor was provided, which was the period between birth and expulsion of the placenta. The umbilical cord was clamped for at least 60 seconds after birth; then 10 IU of prophylactic oxytocin (IM) was administered as the uterotonic of choice for the active management of the third period. The intraumbilical use of oxytocin has been proposed as an efficient procedure to reduce postpartum bleeding. Surveillance of adverse effects after administration of oxytocin was performed, and no adverse effects were reported. All necessary measures were in place to control PPH according to institutional contingency protocols, such as intravenous oxytocin, IM methylergonovine, intrarectal misoprostol, and other measures of the action algorithm.
The study was approved by the Institutional Ethics Committee of the Catholic University of Honduras (#COM-2021-001) and had the institutional approval of the hospital. After approval of the study protocol, patients were actively recruited. All patients provided informed consent before enrollment; institutional permission was obtained to conduct the clinical study under biosafety standards. Experts developed a variable collection form with content validity. The data were entered into collation tables in the IBM SPSS version 25.0 program (IBM Corp., Armonk, NY, USA) (license in force) for subsequent export to the R statistical program (RStudio Team, Boston, MA, USA) where statistical analysis was performed.
Inferential statistics were used to obtain the frequencies and percentages of categorical variables and the medians and interquartile ranges of the quantitative variables. Categorical variables were presented as frequencies and percentages, and quantitative variables as mean and standard deviation (SD) or median with interquartile range (IQR). Comparisons between categorical variables were performed using the chi-square test. Quantitative variables were analyzed using Student’s t-test or Mann-Whitney U test, as needed.
Results
A total of 332 pregnant women were enrolled in the study. They were assigned to the intraumbilical (IU) and IM groups, with 116 patients in each group. The baseline clinical characteristics of the patients were similar between the groups (Table 1).

Baseline clinical and gynecological characteristics in 332 patients with IM and IU oxytocin administration
Schultze’s placental presentation was the most frequent in both groups (83.1%). The median duration of the third stage labor was 0.4 minutes (IQR, 0.25–1.0) in the IU group, which was statistically significantly shorter in the IM group. (P<0.001); 77.1% of the IU group had <1 minute of third-stage labor duration.
The median estimated blood loss was 120 mL (IQR, 80–218.75 mL) for the total sample. There was a statistically significant difference between the groups, showing less estimated blood loss in the IU group with a median of 80 mL (IQR, 60–100 mL) (P<0.001). In the IU group, 92.1% had an estimated blood loss of <150 mL, compared with 33.1% in the IM group. Among the patients in the IM group, 66.8% had an estimated blood loss greater than 251 mL (Table 2).
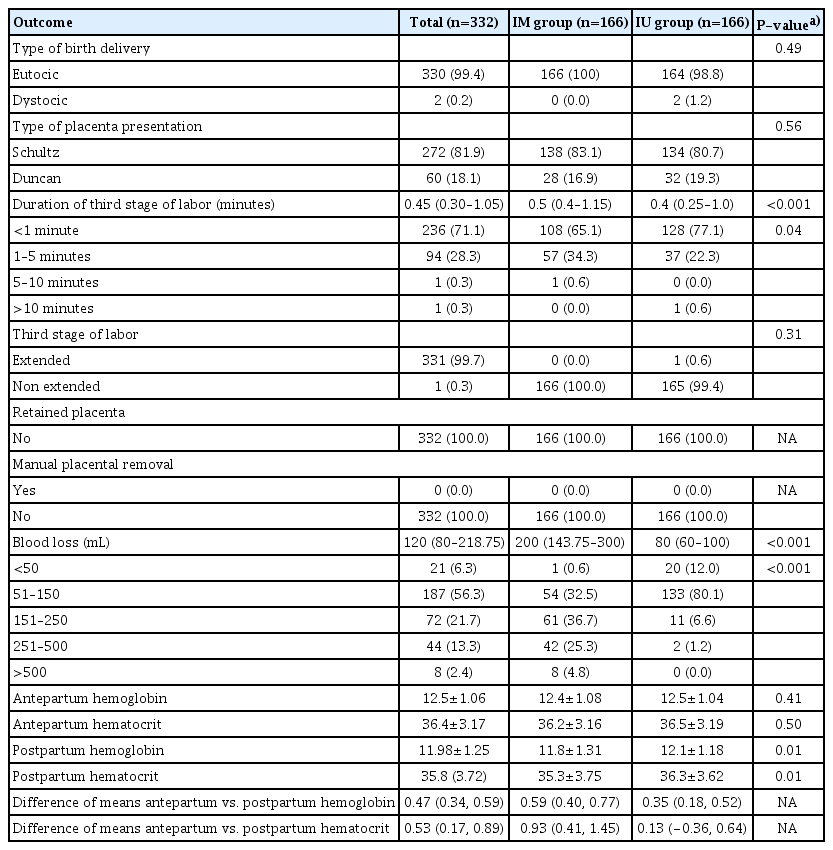
Outcomes of 322 pregnant patients with IU and IM oxytocin administration at the third stage of labor
The mean prepartum hemoglobin for the total sample was 12.45 mg/dL (SD, ±1.06 mg/dL). There was no significant difference between the two groups in prepartum hemoglobin (P=0.41) and hematocrit levels (P=0.50). The total postpartum hemoglobin mean was 11.98 mg/dL (SD, ±1.25 mg/dL). The IU group had a superior mean postpartum hemoglobin (12.14 mg/dL), which was statistically significant compared to the mean (11.81 mg/dL) (P<0.05) for the IM group (Table 2).
Discussion
Delivery is the third stage of labor, which occurs after the expulsion of the fetus. It consists of the detachment and expulsion of the placenta and placental membranes. The conduct to follow is expectant, and its evolution is prophylactically spontaneous; uterotonics such as oxytocin are administered. This cross-sectional study demonstrated that administrating 20 IU of intraumbilical oxytocin injection was more effective than the conventional delivery, with 10 IU of IM oxytocin during the third stage of labor, significantly reducing bleeding and delivery time. The administration through the intraumbilical route is intended to improve the quality of uterine contractions (adequate number, duration, and intensity) essential for placental abruption and proper uterine hemostasis. Intraumbilical administration of oxytocin allows oxytocin to reach the placental bed rapidly in high concentrations, which causes powerful uterine contractions, resulting in placental separation. The resulting pressure causes the separation of the decidua, contributing to the formation of a retroplacental hematoma that accelerates the separation process and, consequently, the decrease in bleeding in the third stage of labor [10,11].
The results showed that the median blood loss in the IU group was 80 mL (IQR, 60–100 mL) and 200 mL (IQR, 143.75–300 mL) in the IM group, highlighting that 66.8% of the IM group had an estimated blood loss greater than 251 mL. These findings are clinically significant, especially in developing countries like ours, where patients have factors that predispose to postpartum bleeding and complications during delivery, including patients with chronic anemia, absence of prenatal controls, absent obstetric ultrasounds, multiparity, early maternal age, low social status, short interpregnancy interval, lack of education and sexual support, among some of the variables to be considered.
The mean postpartum hemoglobin and hematocrit levels in both groups were similar, and no substantial differences were observed. However, there was a notable difference in the volume of blood lost during delivery. Nankali et al. [12] reported similar results; the impact on hemoglobin concentration before and after delivery was not remarkable in their study. The volume of blood lost is a more meaningful and objective parameter for measuring PPH than the estimation of postpartum hemoglobin [1]. In the first instance, intraumbilical oxytocin was proposed as a prophylactic alternative for a retained placenta [13–15], but its significant use in reducing PPH has been demonstrated. Due to the lack of randomized controlled clinical trials with substantial sample sizes that consider the implementation of a placebo, the routine use of intraumbilical oxytocin to prevent PPH is still questionable [9].
Umbilical vein injection for the treatment of retained placenta was first described by Martínez et al. [16]. Since then, it has been studied by different researchers [16], and there is limited published literature evaluating the effect of oxytocin injection into the umbilical vein in routine practices for active management of the third stage of labor to prevent PPH [1]. Our findings are consistent with those reported by other authors [11,17]. Gupta and Athokpam [18] concluded that intraumbilical oxytocin was a better alternative than IM oxytocin in actively managing the third stage of labor. Campero-Maneiro et al. [19] had the same conclusions: reduction of blood loss, delivery time, and placental retention frequency. Puri et al. [20] concluded that intraumbilical injection was a simple, effective, and noninvasive method for actively managing the third stage of labor. Güngördük et al. [1] and Ghulmiyyah et al. [21], showed similar results.
The labor duration in our study was shorter than that reported in other studies, with a median of 0.45 minutes (IQR, 0.30–1.05 minutes) in both groups, and 0.40 minutes (IQR, 0.25–1.0 minutes) and 0.50 minutes (IQR, 0.40–1.15 minutes) in the IM and IU groups, respectively. Sharma et al. [22] reported a labor duration of 1.703 minutes in group IU. Similarly, Nankali et al. [12] showed that women who received oxytocin in the intraumbilical vein had a shorter third stage of labor than the placebo group (4.24±3.27 vs. 10.66±7.41 minutes). Our hospital uses active labor management (prophylactic injection of 10 IU of oxytocin within the 1 minute after birth, early umbilical cord clamping, controlled cord traction, and uterine massage) and early attachment as a regulation of the Ministry of Health to reduce PPH, which significantly reduces the duration of labor; these should be offered and recommended to all women [23].
Any reduction in bleeding during labor is clinically relevant since it improves the patient’s prognosis, especially in those with irregular prenatal care, and its deficiencies are unknown. It is also emphasized that the intraumbilical route is painless, safer, easier, and faster than the IM oxytocin injection, and its prophylactic use during labor is recommended due to the good results obtained and the satisfactory prognosis in the puerperium; its inclusion in hospital protocols would help prevent obstetric hemorrhage and, consequently, would reduce morbimortality due to bleeding and placental retention [11].
The strength of this study was its design. This cross-sectional study assessed this topic in this region. The limitation of this study was the relatively small sample size. Another limitation of our study was that there were no cases of PPH or retained placenta, and our study excluded high-risk cases of PPH. In addition, this was a single-center study. Multicentric studies with a larger population of patients are required for this highly important topic, which could provide an alternative for future protocols.
The use of oxytocin via the intraumbilical route, which is an economical, fast, and safe technique with good outcomes and a satisfactory prognosis during the puerperium in reducing maternal morbimortality, is recommended, and it should be established as an alternative to the strategies established by the Ministry of Health for the management of PPH. More research is needed with studies involving high-risk populations in different institutional settings with different types of delivery, including cesarean sections, which are necessary to confirm these findings.
Notes
Conflicts of interest
The authors declared no competing interests.
Ethics approval
The project was approved by the Institutional Ethics Committee of the Universidad Católica de Honduras (#COM-2021-001) and the hospital.
Patients consent
All patients provided signed informed consent before enrollment; institutional permission was obtained to carry out the clinical study under biosafety standards.
Funding information
The authors declare that this study was not funded by any third parties. All costs were covered by their funds.
Data availability
The patient files and datasets used to support the findings of this study were restricted by the ethics committee of the “Universidad Católica de Honduras” to protect the privacy of clinical data. Data are available to investigators who comply with the criteria for access to confidential data at the request of the ethics committee. Requests for access to these data should be directed to César Alas-Pineda: cesar_alas10@hotmail.com.