Growth inhibition by fusidic acid in cervical, thyroid, and breast carcinoma cell lines
Article information
Abstract
Objective
We investigated the effects of fusidic acid (FA) on human cervical, thyroid, and breast carcinoma cell lines to determine the potential usefulness of FA in cancer treatment.
Methods
Six cancer cell lines (cervical cancer: Caski, HeLa; thyroid cancer: 8505C, TPC1; and breast cancer: MCF-7, MDA-MB-231) were treated with FA. Furthermore the changes in cell growth, cell cycle duration, and extent of apoptosis were analyzed.
Results
After FA treatment, the cancer cells showed a decrease in growth rate. In the cell death assay, the cell populations were similar in each cell type after treatment with FA, indicating that growth inhibition by FA was not related to the induction of apoptosis. FA induced cell cycle arrest at a dose that inhibited growth rate, which varied in different cell types. G0/G1 phase arrest occurs in breast cancer, S phase arrest in 8505C thyroid cancer, and G2/M phase arrest in cervical cancer. These results indicate that FA reduces growth rates by inducing cell cycle arrest.
Conclusion
FA treatment can interfere with cell proliferation by inducing cell cycle arrest in human cervical, thyroid, and breast carcinoma cell lines. Thus, FA can be useful in treating human cervical, thyroid, and breast carcinomas.
Introduction
Cervical cancer is the 10th most common cancer in Korea, accounting for 2.7% of newly diagnosed cancers in women; a total of 3,469 new cases occurred in 2017 [1]. Breast cancer is the most common cancer (20.6%), followed by thyroid cancer (19.2%). The combined incidence of thyroid, breast, and cervical cancers is 42.5% [2].
Fusidic acid (FA) is an oral antistaphylococcal antibiotic that has been used in Europe for more than 40 years to treat skin, chronic bone, and joint infections [3]. It is a steroidal antibiotic that prevents protein synthesis by targeting ribosome-bound elongation factor G (EF-G) for both translocation and ribosome recycling [4]. FA has been used to treat infections caused by gram-positive bacteria since its discovery in the early 1960s [5]. A previous study suggested that FA could be applied to cancer cells by inhibiting the thromboplastic action of cancer coagulative factor (CCF) contained in human cancer cells and found that it had some effect on breast cancer; however, no definite conclusions could be drawn from such a small series. The secondary effects of blocking protein synthesis include interruption of RNA and DNA synthesis [6].
In this study, we sought to investigate the anti-cancer effects of FA on human cervical, thyroid, and breast carcinoma cell lines and determine whether FA is capable of cell cycle arrest.
Materials and methods
1. Cell culture
Six cancer cell lines (breast cancer cell lines: MCF-7, MDA-MB-231; thyroid cancer: 8505C, TPC1; cervical cancer: Caski, HeLa) were used for this study. Cancer cell lines were cultured in Dulbecco’s modified eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). For adherent culture, 5×105 cells were grown in tissue culture dishes. All the cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2.
2. FA treatment and growth rate
The cells were seeded in DMEM containing 10% FBS. After 24 hours, the cells were washed twice with phosphate-buffered saline (PBS), and fresh medium was added. Cells were treated for 72 hours with distilled water and 10 μM, 100 μM, and 200 μM FA (Sigma, St. Louis, MO, USA). After treatment, the cells were harvested for growth rate analysis. Growth rate was estimated as the number of positively stained (using 0.4% trypan blue dye) surviving cells in a Newbauer chamber.
3. Cell cycle analysis
After treatment with distilled water and 10 μM, 100 μM, and 200 μM FA for 72 hours, the cells were collected. Cells were fixed in 70% ethanol for 25 minutes, washed with PBS, treated with 100 μg/mL Ribonucleases a for 90 minutes at 37°C, and stained with 10 μg/mL propidium iodide (PI). Flow cytometry was performed thrice for each experiment using a FACSCalibur system (BD Biosciences, Franklin Lakes, NJ, USA).
4. Annexin V staining
Using Annexin V staining for apoptosis analysis, the cells were washed with PBS, treated with a diluted trypsin-ethylenediaminetetraacetic acid solution, centrifuged, washed twice with cold PBS, and resuspended in a binding buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2). A 100-μL aliquot of the suspension (1×105 cells) was cultured with 5 μL Annexin V-fluorescein isothiocyanate (FITC) and 5 μL PI for 15 minutes at room temperature in the dark. Binding buffer (400 μL) was added to each mixture, and the samples were analyzed using flow cytometry within 1 hour. Flow cytometry was conducted using the FACSCalibur system, and all experiments were performed three times.
5. Statistical analyses
At least three iterations were performed for all analyses. Data are presented as the mean±standard error. Student’s t-test was used to compare results between treated and control cells. Statistical significance was set at P<0.05.
Results
1. Growth rates of breast, thyroid, cervical cancer cells after FA treatment
To observe the anti-cancer effect of FA treatment on growth rates in breast, thyroid, and cervical cancer cells, cells were treated with FA in a dose-dependent manner (10 μM, 100 μM, and 200 μM) for 72 hours. In a breast cancer cell line, the inhibitory effect was greatest at 100 μM, and the growth rate decreased by 28% in MCF-7 and 32% in MDA-MB- 231 cells. In the thyroid cancer cell line, 8505C had the greatest inhibitory effect at 200 μM (43%), and TPC1 had the greatest inhibitory effect at 100 μM (45%) (Fig. 1B). In the cervical cancer cell line, the inhibitory effect was the greatest at 200 μM, and the growth rate decreased by 29% at Caski and 38% at HeLa (Fig. 1C).
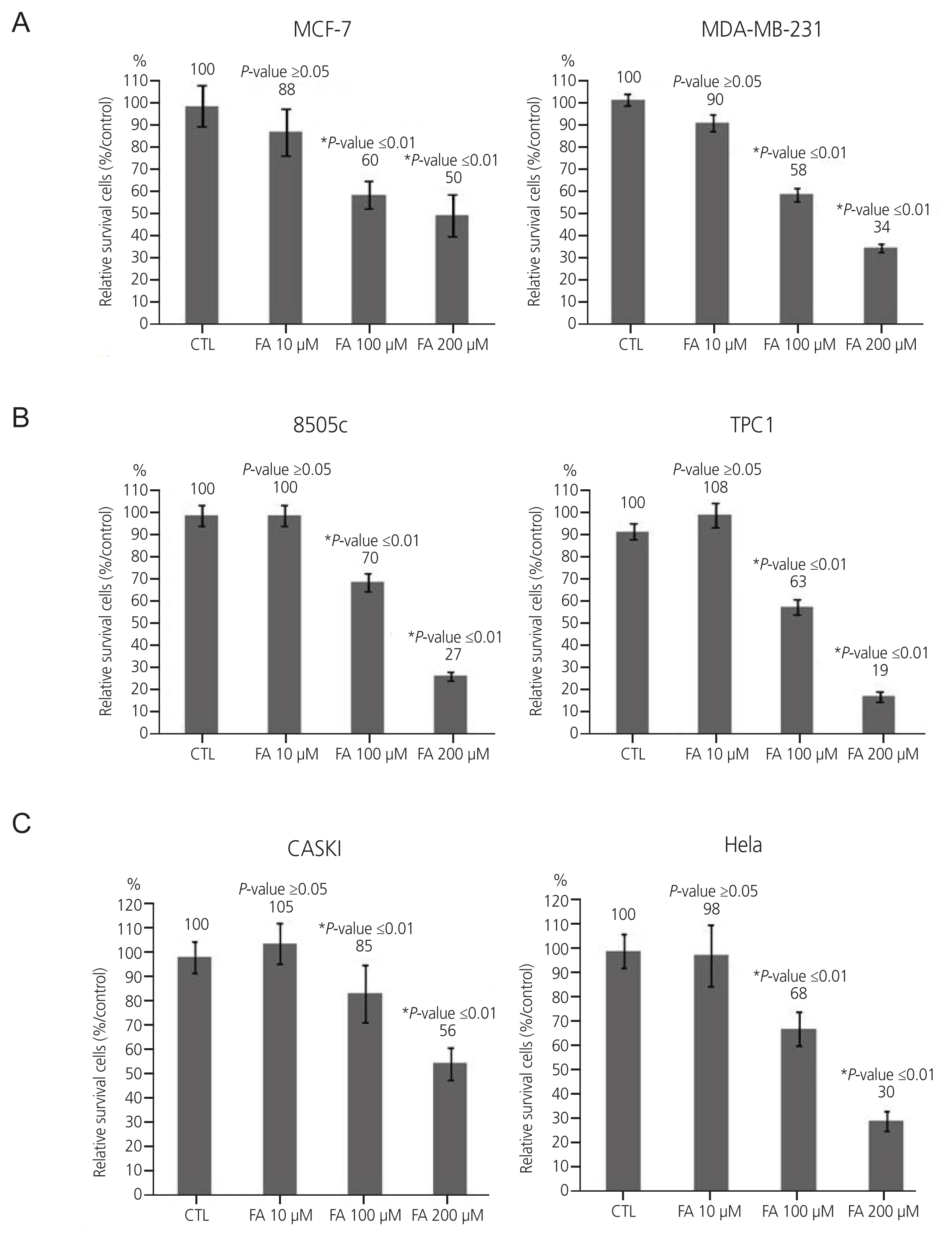
Effect of FA on six cancer cell lines (breast cancer cell lines: MCF-7, MDA-MB-231; thyroid cancer: 8505C, TPC1; cervical cancer: Caski, HeLa) were treated with FA at 10 μM, 100 μM, and 200 μM. CTL, control; FA, fusidic acid. *A significant inhibitory effect was shown at the concentraion of 100 μM and 200 μM in breast cancer cancer cell lines, 100 μM and 200 μM in thyroid cancer cell lines, and 200 μM in cervical cancer cell lines respectively.
2. Death of breast, thyroid, and cervical cancer cells after FA treatment
FA decreased the growth rate of the cancer cells after 72 hours of treatment (Fig. 1). To investigate this decrease in growth rate, we analyzed cell death after Annexin V and PI staining, followed by flow cytometry. Using Annexin V staining for apoptosis analysis, we detected an increase in the survival fraction (2%) at 200 μM in MDA-MB-231 cells but a slight decrease (1%) at 200 μM in Annexin V-FITC-positive or PI-negative cells. The latter refers to early apoptotic cells and a survival fraction increase (7%) at 200 μM in MCF-7 cells but decreased (4%) at 200 μM in Annexin V-FITC-positive or PI-negative cells (Fig. 2A). We detected an increase in the survival fraction (1%) at 200 μM in 8505C cells, but a slight decrease (2%) at 200 μM in Annexin V-FITC-positive or PI-negative cells, and a survival fraction increase (3%) at 200 μM TPC1 cells but decrease (3%) at 200 μM in Annexin V-FITC-positive or PI-negative cells (Fig. 2B). Our analysis of the Caski cells showed a slight decrease (1%), and HeLa cells showed a slight increase (2%) in the Annexin V-FITC-positive cells or PI-negative cells (Fig. 2C). These results show that growth inhibition by FA was not related to the induction of apoptosis.
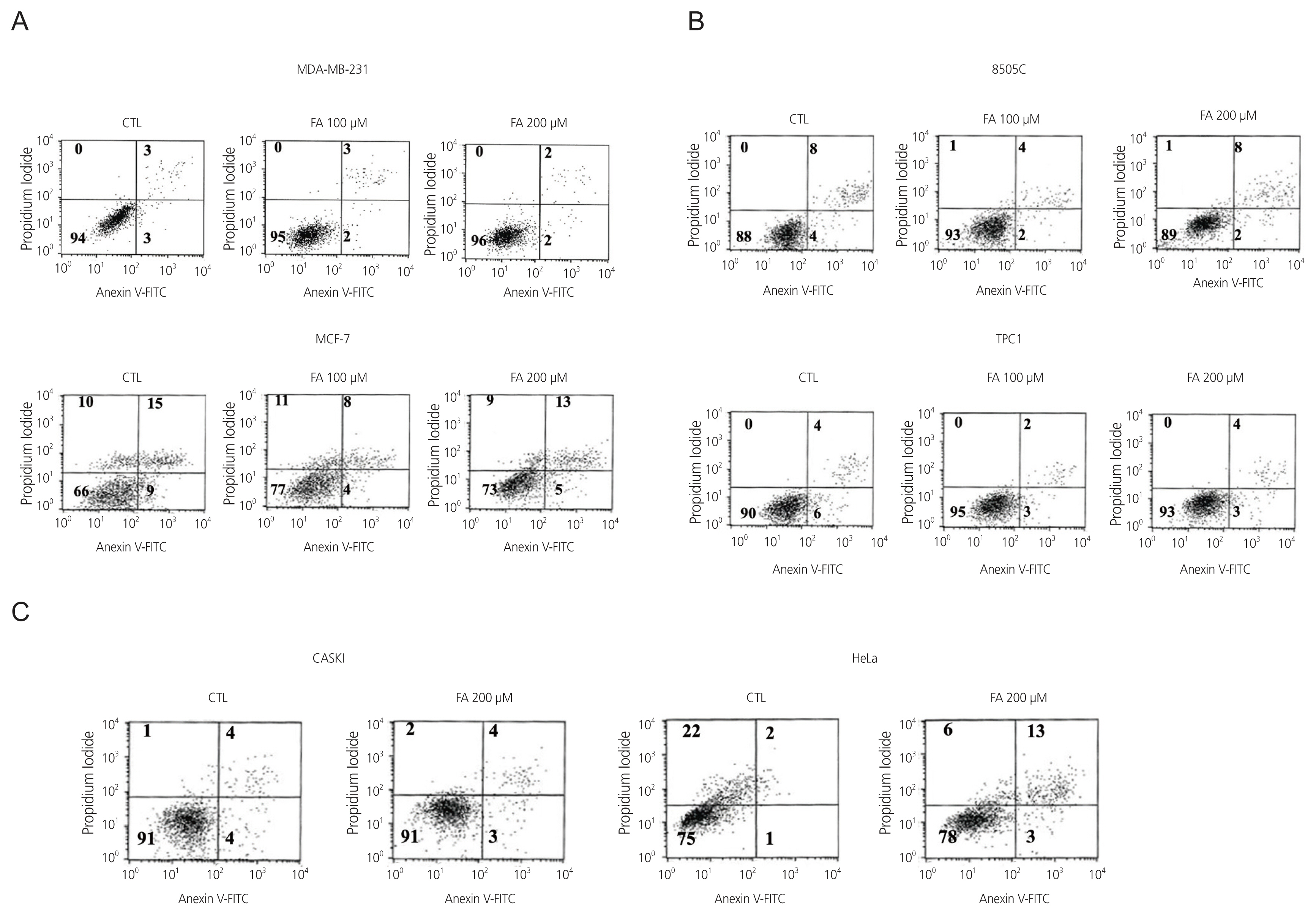
Cell death analysis was done after treatment of six cancer cell lines with FA at 100 μM and 200 μM through Annexin V and PI staining followed by flow cytometry. (A) breast cancer cells (MCF-7, MDA-MB-231), (B) thyroid cancer cells (8505C, TPC1), (C) cervical cancer cells (Caski, HeLa). CTL, control; FA, fusidic acid; FITC, fluorescein isothiocyanate; PI, propidium iodide.
3. Cell cycle progression in breast, thyroid, and cervical cancer cells after FA treatment
We did not observe any cell death effects of FA, despite the induced inhibition of the growth rate (Fig. 1). To confirm the effects of FA on cell death, the cell cycle was analyzed by PI staining followed by flow cytometry after FA treatment. FA induced cell cycle arrest at a dose that inhibited the growth rate; however, the phases of cell cycle arrest were different among cancer cell types (Fig. 3). G0/G1, S, and G2/M phase arrest occurred in breast, thyroid, and cervical cancer cell lines, respectively (Fig. 3). MDA-MB-231 cells showed an increase (16%) in DNA content, and MCF-7 cells showed an increase (42%) in DNA content at 200 μM during the G0/G1 phase (Fig. 3A). The DNA content in 8505C and TPC1 cells increased to 12% and 7%, respectively, at 200 μM during the S phase (Fig. 3B); Caski cells showed an increased (11%) DNA content at 200 μM during the G0/G1 phase; and HeLa cells showed an increase (15%) in DNA content at 200 μM during the G2/M phase (Fig. 3C). These results show that growth inhibition by FA was not related to the induction of apoptosis.

Six cancer cells were treated with the indicated amounts of FA at 100 μM and 200 μM for 72 hours and the percentages of cells at each stage of the cell cycle were analyzed by flow cytometry after staining the DNA with PI. (A) Breast cancer cells (MCF-7, MDA-MB-231), (B) thyroid cancer cells (8505C, TPC1), (C) cervical cancer cells (Caski, HeLa). CTL, control; FA, fusidic acid; PI, propidium iodide.
Discussion
FA is primarily active in vitro against various strains of Staphylococci, including methicillin-susceptible and methicillin-resistant Staphylococcus aureus (S. aureus), heterogeneous and non-heterogeneous vancomycin-intermediate S. aureus, and most coagulase-negative Staphylococcus and Clostridia species. Peptococcus, Neisseria, Moraxella, and Legionella pneumophila are gram-negative bacteria susceptible to FA [7]. FA is derived from the fungus Fusidium coccineum, and its structure was determined by Godtfredsen et al. [5] and Arigoni et al. [8] in the 1960s.
FA inhibits protein synthesis in vitro and in vivo [9]. FA binds to elongation EF-G in ribosomes to prevent bacterial protein synthesis, thereby blocking the release of EF-G guanosine diphosphate complex and delaying bacterial protein synthesis by inhibiting translation. The antibacterial action of FA is mainly bacteriostatic; however, at high concentrations, it may be bactericidal. The gene encoding EF-G is FusA, which is found in S. aureus [10]. Both cell cycling and protein synthesis are major physiological tasks in cancer cells. The elongation step in protein synthesis, controlled by elongation factor 2 (EF2) and EF2 kinases, may provide clues regarding the origin and maintenance of cancer cells. Protein elongation mechanisms are closely related to cell cycle progression such that dysregulation of EF2/EF2 kinase can alter the cell cycle and vice versa. EF2 or EF2 kinase may be overexpressed in cancer cells to regulate their influence on the cell cycle. EF2 plays a role in the mechanisms of action of well-established drugs. For example, doxorubicin (DOX) is one of the oldest and most commonly used chemotherapeutic drugs. The recently described effect of EF2 is particularly significant [11]. Upon treatment with DOX, EF2 is strongly phosphorylated, protein translation is disrupted, cells are arrested at the G2/M phase, and stores of short-half-life proteins with antiapoptotic properties are rapidly depleted, making cells more susceptible to death. Another commonly used drug is taxol, which is widely considered to act by stabilizing microtubules, thereby arresting cycling cells in the G2/M phase [12]. In addition, it was recently observed that exposure to taxol resulted in persistent phosphorylation of EF2 via an unknown mechanism [13].
Therefore, the action of EF-inhibitor FA could affect cancer cells as an extension of this mechanism. We focused on the anti-cancer effects of FA on cancer stem cells in six cell lines (two breast cancer, two thyroid cancer, and two cervical cancer cell lines). To study the anti-cancer effects of FA in these cells, we hypothesized that FA could induce a decreased growth rate in cancer stem cells due to their ability to differentiate. For this purpose, we evaluated the growth rate, side population cell ratio, cancer stem cell markers, epithelialmesenchymal transition markers, and FA signaling molecules in cancer cells following FA treatment. FA induced a decrease in growth rate. FA inhibits cell growth through cell cycle arrest.
These results show that FA exerts anti-cancer effects by inhibiting the growth of cancer cells. Growth inhibition by FA is mediated by cell cycle arrest and is not associated with apoptosis in cells. FA induced cell cycle arrest at a dose that inhibited growth rate but this varied by cell type. FA induces G0/G1 phase arrest in breast cancer, S phase arrest in 8505C thyroid cancer, and G2/M phase arrest in cervical cancer.
A study published in 1966 suggested that FA is an effective cancer treatment. Human cancer cells contain a labile thromboplastic substance called CCF [13,14]. As FA is an effective treatment against coagulase-positive staphylococci through the inhibition of CCF. thromboplastic action, 13 advanced cases were studied under the assumption that this principle could be applied to cancer cells. The results obtained suggest that fucidin has some effect on breast carcinoma; however, no definite conclusions could be drawn from such a small series. Since then, no studies have used FA for cancer treatment. Our study showed a different mechanism than that of Thornes, which was published in 1966 [14]. By confirming that FA inhibits protein synthesis, thereby arresting the cell cycle and lowering the growth rate, it is possible to suggest FA as a potential cancer treatment.
However, we performed limited experiments with thyroid, breast, and cervical cancers, and while the mechanism of cell cycle arrest has been postulated, the exact mechanism has not been identified. For FA to be used in cancer treatment, it must be applied to more types of cancer cells. The commonalities and differences between cancer cells should be determined and an accurate mechanism should be proposed.
To use FA as an anti-cancer drug, an issue needs to be overcome. FA occasionally causes liver damage that may cause jaundice. This condition will almost always improve after the patient completes taking fucidin. Other side effects include dark urine and lighter-than-usual feces [15].
In conclusion, FA treatment inhibits cancer cell proliferation through cell cycle arrest. FA could be a novel anti-cancer drug for the treatment of cervical, breast, and thyroid cancers.
Notes
Conflict of interest
The author declare no conflicts of interest.
Ethical approval
Not applicable.
Patient consent
Not applicable.
Funding information
The authors did not receive support from any organization for the submitted work.
