Ritodrine in external cephalic version: is it effective and safe?
Article information
Abstract
Objective
The external cephalic version (ECV) has been shown to lower the likelihood of cesarean section requirements among pregnant women with breech presentations. In the current study, we investigated the effectiveness and safety of ritodrine as a tocolytic for ECV.
Methods
A total of 407 pregnant women with breech presentations, who had no contraindications for ECV, were enrolled in this study. Multivariable logistic regression analyses were used to assess the impact of ritodrine use on the safety and efficacy of ECV.
Results
The overall success rate was 67.6%, and ritodrine use was associated with significantly higher odds of successful ECV after adjusting for confounders. Moreover, using ritodrine did not increase the risk of adverse effects, including temporary changes in fetal heart rate, need for elective or emergency cesarean section due to fetal distress during ECV, low Apgar scores, and perinatal mortality.
Conclusion
Our results suggest that using ritodrine as a tocolytic during ECV may increase the likelihood of ECV success and may not increase adverse perinatal outcomes.
Introduction
The rate of cesarean sections in Korea has increased sharply since 1980. The most recent incidence of cesarean sections in Korea is estimated to be 40%, the highest in the world [1]. However, cesarean section has increased the risk of complications in the mother and fetus [2]. The ideal rate of cesarean sections is estimated to be 10–15% because there is no improvement in maternal and newborn mortality rates beyond this [3]. Therefore, a cesarean section should be considered only when the benefits outweigh the risks [4].
The external cephalic version (ECV) is a manual procedure that rotates a breech fetus to the vertex to ensure vaginal delivery. It is a relatively simple and safe procedure that does not require complicated intervention [5,6]. In addition, it can reduce the likelihood that a cesarean section will be required for breech presentation.
However, ECV is not always successful. Several studies have investigated ways to improve the results of ECV, including tocolysis [7–11].
In terms of tocolysis for successful ECV, some studies have shown that beta 2-adrenergic receptor agonists, including ritodrine and salbutamol for tocolysis improved success rates in nulliparous or multiparous patients [7–9]. Other studies have shown that atosiban, nifedipine, and nitroglycerin can be used to improve the success rates of ECV [10–12].
Among these tocolytics, ritodrine is the most effective and safe for successful ECV treatment. However, there are no reported data on the safety and effectiveness of ritodrine for ECV treatment in Korea. Therefore, we occasionally encountered pregnant women who refused the application of ritodrine for successful ECV despite its advantages. In the current study, we examined the safety and effectiveness of ritodrine as a tocolytic agent during ECV in pregnant Korean women [7].
Materials and methods
This study was approved by the Institutional Review Board of Inje Medical University, Goyang, Korea. Written informed consent was obtained from all the participants.
1. Patient selection
From 2014 to 2021, 407 pregnant women with breech presentations, without any contraindications to ECV, presented to Cheil General Hospital & Women’s Healthcare Center (n=399, 2014–2019) and Ilsan Paik Hospital (n=8, 2020–2021). The use of ritodrine for successful ECV is recommended for all pregnant women. However, some pregnant women refused ritodrine because of concerns regarding fetal safety. The study participants included 358 ritodrine users in the research group and 49 non-users in the control group (Fig. 1). The following exclusion criteria were applied: active labor, rupture of membranes, vaginal bleeding, severe preeclampsia or eclampsia, multiple gestations, major fetal abnormalities, abnormal cardiotocography, and previous cesarean section [5]. All candidates for ECV were interviewed for baseline characteristics (maternal age, gravidity, parity, height, weight, and history of other gestational diseases). Additionally, fetal sonography was performed to check fetal weight, position, amniotic fluid index (AFI), location of the placenta, presence of myoma, and umbilical artery Doppler index.
2. Procedure details
The procedure was as follows: tocodynometry was performed approximately 30 minutes to 2 hours before ECV to check fetal heart rate and uterine contraction. In the tocolysis group, intravenous ritodrine was administered (100 mg in 50 g dextrose) as an initial intravenous dose of 1.6 mg/h for tocolysis for 1 hour. The main surgeon pushed the fetal buttock upward from the maternal pelvis with one hand while the other hand pushed the fetal head downwards. During the ECV procedure, the first assistant performed a real-time sonogram to ensure the fetal heart rate and direction of the fetal head. The second assistant also provided assistance during the ECV procedure, when required. The procedure was aborted if the patient expressed severe abdominal pain or if the fetal heartbeat demonstrated an abnormal pattern. In such cases, ECV was reattempted after allowing the candidate to rest and for the fetal heart rate pattern to return to normal. After the procedure, fetal monitoring was performed for at least 3 hours to ensure optimal fetal conditions.
3. Assessment of effectiveness and perinatal outcomes by ritodrine use during ECV
The effectiveness of ritodrine during ECV was assessed by comparing the success rate of ECV between the two groups. We evaluated perinatal outcomes by comparing the rates of adverse outcomes, including temporary changes in fetal heart rate and emergency cesarean section, due to fetal distress during ECV. Additionally, we evaluated the overall rate of cesarean section, Apgar scores at 1 minute and 5 minutes, and perinatal mortality.
4. Statistical analysis
We evaluated the fetal and neonatal safety and the efficacy of ECV in patients with and without ritodrine administration. Using SPSS version 26.0 (IBM, SPSS Statistics, Armonk, NY, USA), univariate analyses were performed using chi-square test or Fisher’s exact test for categorical data and Student’s t-test or Wilcoxon test for continuous data. The efficacy of ritodrine (successful ECV) was evaluated using a multivariate logistic regression analysis after adjusting for confounders. The statistical significance was set at P<0.05.
Results
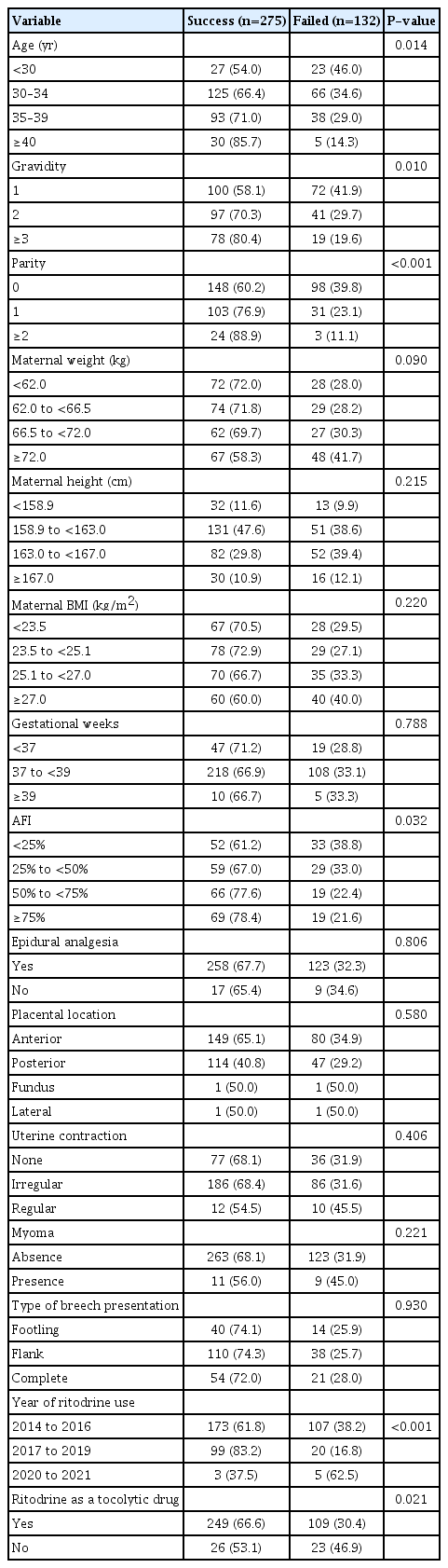
ECV was attempted in 407 pregnant women with breech presentations and was successful in 275 (67.5%). On comparing the characteristics between patients with and without ritodrine administration, we did not observe any significant differences in baseline characteristics, except for uterine contraction (P=0.05), year of ritodrine use (P<0.001), and the presence of uterine myoma, which was found to be more frequent among patients without ritodrine administration compared to those with ritodrine administration (12.2%, n=6 vs. 3.9%, n=14; P=0.012) (Table 1).
Univariate analysis showed that patients administered ritodrine had a significantly higher ECV success rate (66.6%, n=238 vs. 53.1%, n=26; P=0.021). Additionally, maternal age (P=0.014), gravidity (P=0.010), parity (P=0.001), AFI (P=0.032), and year of ritodrine use (P<0.001) were associated with ECV success (Table 2).
On multivariable logistic analysis, ritodrine administration was associated with higher odds of ECV success (odds ratio, 2.755; 95% confidence interval [CI], 1.170–6.488; P=0.020) after adjusting for age, gravidity, parity, AFI, uterine contraction, and year of ritodrine use (Table 3).

Multivariate logistic regression analysis for ritodrine as a successful predictive factor of external cephalic version
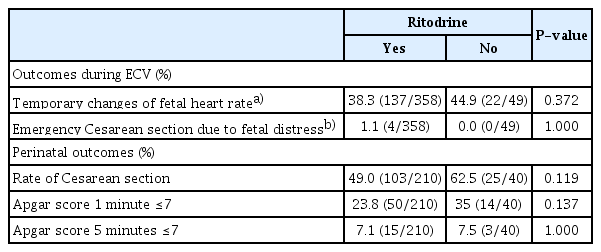
There were no significant differences between the two groups in the rate of adverse outcomes, including temporary changes in fetal heart rate and emergency cesarean section due to fetal distress during ECV. Similarly, we did not observe any significant differences between the two groups regarding adverse perinatal outcomes, including the overall rate of cesarean section and Apgar scores at 1 minute and 5 minutes (Table 4). No perinatal mortality was observed in either group.
Discussion
In the current study, the use of ritodrine as a tocolytic during ECV was significantly associated with higher odds of successful ECV after adjusting for age, gravidity, parity, and AFI. We adjusted for these variables because they have previously been shown to impact ECV outcomes [5,13,14]. Our results are similar to those reported in previous studies investigating the use of ritodrine in ECV [7,8,15]. In a prospective controlled randomized, double-blind trial by Nor Azlin et al. [8] ritodrine was effective in both nulliparous (34.4% vs. 13.0%) and multiparous women (87.5% vs. 57.1%). Cluver et al. [15] performed a meta-analysis of 28 clinical trials to investigate the interventions and factors associated with the improved odds of ECV success. They found that ritodrine was more effective than placebo (relative risk 1.68; 95% CI, 1.14–2.48).
The mechanism of ritodrine as a tocolytic agent has been previously described. The drug binds to beta-2 adrenergic receptors on the myometrium. This binding increases the levels of intracellular cyclic adenosine monophosphate, which decreases the intracellular calcium concentrations. During uterine contraction, calcium combines with calmodulin, and this complex activates myosin lignin chain kinase, which causes the sliding of myosin over actin fibers. As a result, lowering calcium levels weaken the uterine muscle contractions [16,17]. Therefore, it is reasonable to suggest that decreased uterine muscle contraction may be associated with successful ECV. Another important finding in our study is that ritodrine is safe during ECV, as evidenced by the similar rates of adverse perinatal outcomes between the two groups. This finding is consistent with the results of previous studies [5,6].
In a review article, Kim [5] reported that a large study that included 4,117 patients showed that an ECV trial at term was not associated with increased prenatal morbidity or mortality after comparison with expectant management. Therefore, the latest guideline (2017) issued by the Royal College of Obstetricians & Gynaecologists concerning ECV does not recommend standard preoperative preparations for cesarean section in women undergoing ECV. Collins et al. [6] reported complications from 805 consecutive ECV attempts. They reported an extremely low rate of complications, including ECV-attributable mortality (0/805), abnormal fetal cardiogram (13/805), emergency cesarean section (4/805), and an Apgar score of less than 7 at 5 mintues (3/800) after the procedure. In addition, two studies showed that ritodrine might decrease the probability of emergency cesarean section [7,8]. Impey and Pandit [18] found no significant differences in Apgar score at 5 minutes, mean arterial pH, admission to the neonatal intensive care unit, and neonatal seizures between the ritodrine and non-ritodrine groups. Additionally, ritodrine has been found to have a reasonably safe profile in terms of adverse effects. It is not associated with chest pain, dyspnea, tachycardia, palpitation, tremor, headaches, hypokalemia, hyperglycemia, and pulmonary edema [16]. A key reason for this safety profile is the short duration of ritodrine administration.
This study had some limitations. First, our results cannot be generalized because of the small sample size. Second, this was an observational study; therefore, there might be some selection bias, even though there were no significant differences in basic characteristics, including age, gravidity, parity, and maternal body mass index between the two groups in the study. Additionally, there may be some selection bias caused by non-random sampling, given that participants in the ritodrine use (research) and non-use (control) groups are up to their voluntary decisions after obtaining information on the advantages of ritodrine use during ECV. In addition, a smaller portion (12%, 49/409) of the control group may have reduced the power and meaning of the study.
Finally, given that this study was conducted at two institutions in Korea, there may be a lack of generalizability to other races and ethnic groups. Therefore, caution should be exercised when applying this conclusion to other ethnicities. It is important to note that the sample size in our study was relatively large compared to previous studies and sufficiently powered. Thus, we are confident that our results, combined with those of previous studies, demonstrate the safety and efficacy of ritodrine in patients with breech presentation undergoing ECV. In conclusion, our results suggest that using ritodrine as a tocolytic during ECV may increase the likelihood of ECV success and may not increase adverse perinatal outcomes. Further studies with larger sample sizes and randomizations are needed.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
Research involving human subjects complied with all relevant national regulations and institutional policies and is by the tenets of the Helsinki Declaration (as revised in 2013), and has been approved by the Institutional Review Board of Inje Medical University (IRB No. 2021-09-005-001).
Patient consent
Informed consent was obtained from all individuals included in this study.
Funding information
None.