Sonographic evaluation of fetal scrotum, testes and epididymis
Article information
Abstract
External male genitalia have rarely been evaluated on fetal ultrasound. Apart from visualization of the penis for fetal sex determination, there are no specific instructions or recommendations from scientific societies. This study aimed to review the current knowledge about prenatal diagnosis of the scrotum and internal structures, with discussion regarding technical aspects and clinical management. We conducted an article search in Medline, EMBASE, Scopus, Google Scholar, and Web of Science databases for studies in English or Spanish language that discussed prenatal scrotal pathologies. We identified 72 studies that met the inclusion criteria. Relevant data were grouped into sections of embryology, ultrasound, pathology, and prenatal diagnosis. The scrotum and internal structures show a wide range of pathologies, with varying degrees of prevalence and morbidity. Most of the reported cases have described incidental findings diagnosed via striking ultrasound signs. Studies discussing normative data or management are scarce.
Introduction
Prenatal diagnosis of genital anomalies involves evaluation of fetal genitalia. Traditionally, it has been given little importance beyond fetal sex determination. Moreover, there are no associated guidelines or specific recommendations by scientific societies [1–3]. Considering this scant attention, the scrotal sac and its contents are one of the least studied fetal structures. A probable reason is that testicular descent does not occur until the 24th week of gestation, leading to a missed opportunity to review them in the mid-trimester ultrasound [4,5]. However, the testes play an important role in male fetal development and there are several significant pathologies that can be diagnosed during pregnancy including meconium periorchitis (MPO), intrauterine testicular torsion (IUTT), inguinoscrotal hernia, or cryptorchidism, which is the most common congenital anomaly in male newborns [6–10]. The objective of this study was to review the current knowledge about prenatal diagnosis of pathologies related to the scrotum and internal structures, with discussion regarding ultrasonography, morphometry, pathology, and clinical management.
Methods
We conducted an article search in the Medline, EMBASE, Scopus, Google Scholar, and Web of Science databases for original studies, reviews, or clinical case reports that discussed the prenatal diagnosis of pathologies related to the scrotum, testes, and/or epididymis. A combination of relevant Medical Subject Headings terms, keywords, and word variants for “fetal scrotum,” “fetal testes,” “fetal medicine,” and “prenatal ultrasound” were used. We included articles published between 2000 and 2021. Studies published in languages other than English or Spanish were excluded. Two expert authors independently evaluated the articles.
Results
We identified 72 studies that met the inclusion criteria. We have synthesized all the relevant information and data in the following sections: embryology, ultrasound, pathology, and prenatal diagnosis.
1. Embryology
During the early stages of organogenesis, three basic structures are formed: bipotential gonads, mesonephric cords, and undifferentiated external genitalia including the phallus and the labioscrotal folds. Presence of the SRY gene on the Y chromosome initiates male sex determination and testosterone production. Its androgenic influence results in a differentiation process wherein the gonads form the testicles; the mesonephric cords form the epididymis, vas deferens, and seminal glands; phallus elongates and differentiates into penis; and the labioscrotal folds fuse to form the scrotum [11,12].
Testicular migration begins in the 8th week of gestation and continues until the 35th week [13,14]. It consists of two distinct stages. The first occurs in the abdominal region and is based on the difference between linear fetal growth and the traction exerted by the gubernacular bulb anchored in the scrotum. This stage occurs between the 10th and 15th weeks and is hormonally governed by insulin-like hormone 3 produced by the Leydig cells [15,16]. The second inguinal stage is mainly controlled by androgens through their effect on masculinization of the femoral-inguinal nerve [16]. It starts in the 20th week and is usually a relatively quick process that lasts for 3 to 4 weeks [14].
2. Ultrasound
To the best of our knowledge, there seems to be no detailed description in the medical literature about fetal ultrasound of the scrotum and its internal structures. The only study on technical aspects is by Fait et al. [17], which discussed correct visualization of the scrotum and testicles in late pregnancy. Ultrasound exploration is usually possible in 100% of the patients between 34th and 36th weeks of gestation and the possibility gradually declines to 71.4% in the 40th week. Factors that prevent optimal visualization of the scrotum include decreased amniotic fluid and unfavorable fetal position. In contrast, postnatal scrotal ultrasound is a fully standardized technique that is widely used in pediatrics and urology [18–20].
There is a very limited amount of literature on morphometric measurements of male genital growth throughout pregnancy except the examination of fetal penis. Two studies measured the scrotum using different techniques. Achiron et al. [4] performed a measurement of the scrotal circumference from the outer edge. Pinette et al. [21] opted for a transverse measurement at the widest point and also from edge to edge. Only one study has been conducted on testicular size [22]. Rotondi et al. [22] used the maximum testicular diameter obtained from the 25th to the 40th week. Other anatomical studies combined two or more measures among length, width, thickness, or weight [23,24]. Postnatal scrotal ultrasound allows the evaluation of all of the aforementioned measures including testicular volume [25]. All of these studies have reported a high correlation between anatomical measurements and gestational age (Table 1) [4,21–24].
Important ultrasound findings include the absence of descended testicles, morphological abnormalities, and presence of scrotal masses. Testicular descent is the main qualitative change that can be assessed throughout pregnancy. There are several publications that have reviewed testicular descent using ultrasound [4,5,7,17,26], magnetic resonance [27], or anatomical studies [28]. All of these studies agree that descent begins at 24th to 25th week in 5% of the cases and ends at 32nd to 33rd week in 97% of the fetuses [4,7,17]. The remaining 3% of the cases correspond to undescended testicles or cryptorchidism [17]. The descent is usually bilateral, with less than 6% asymmetry and the right testicle seems to be the first to complete the migration [28]. Morphological abnormalities include alterations in the position, number, or form of the scrotum or testicles. These include bifid scrotum, scrotoschisis, or penoscrotal anomalies [29–31]. Scrotal masses can exhibit all types of morphologies including solid or cystic appearance, calcification, peristaltic movement, or presence of blood flow on Doppler ultrasound. They could correspond to testicular or paratesticular tumors such as teratomas, rhabdomyosarcomas, hemangiomas, lymphomas, or metastases. Other abnormalities such as hydrocele, hematocele, meconial ascites, pseudocysts, inguinoscrotal hernia, or testicular torsion could also be observed [32–34].
3. Pathology
There are many pathologies with varying degrees of frequency (Table 2). Although they may be interrelated, we have grouped the entities according to the main structures involved.
1) Scrotal pathologies
We have included morphological anomalies as well as the presence of scrotal masses.
4. Morphological abnormalities
Scrotal morphological pathologies include a group of rare anomalies whose etiology is not fully understood. However, the main theory is that they occur due to disruption during early division and migration of the labioscrotal folds [35,36]. The main recognized entities include scrotal transposition, bifid scrotum, accessory scrotum, and scrotal agenesis as well as other minor ones such as central scrotalization of the middle raphe or wide penoscrotal distance (Figs. 1 and 2) [31]. One of the main issues is the presence of associated anomalies. Accessory scrotum and ectopic scrotum are usually associated with contiguous subcutaneous tumors [37,38], bifid scrotum is usually associated with scrotal or perineal hypospadias [39], and the most serious cases such as penoscrotal transposition are usually associated with severe malformations of the genitourinary, intestinal, cardiovascular, and skeletal systems [36,40–42]. Scrotal abnormalities, especially the bifid scrotum, may occur due to undervirilization, since lip fusion is governed by androgens. Therefore, it could represent a disorder of sex development (DSD) [43,44]. Mild and isolated defects such as accessory scrotum or scrotal agenesis have a good prognosis and treatment could be surgical or even conservative with topical androgen creams [45–47]. More serious defects such as complete transposition require corrective surgery as well as a complete assessment of the rest of the anomalies [36,48].
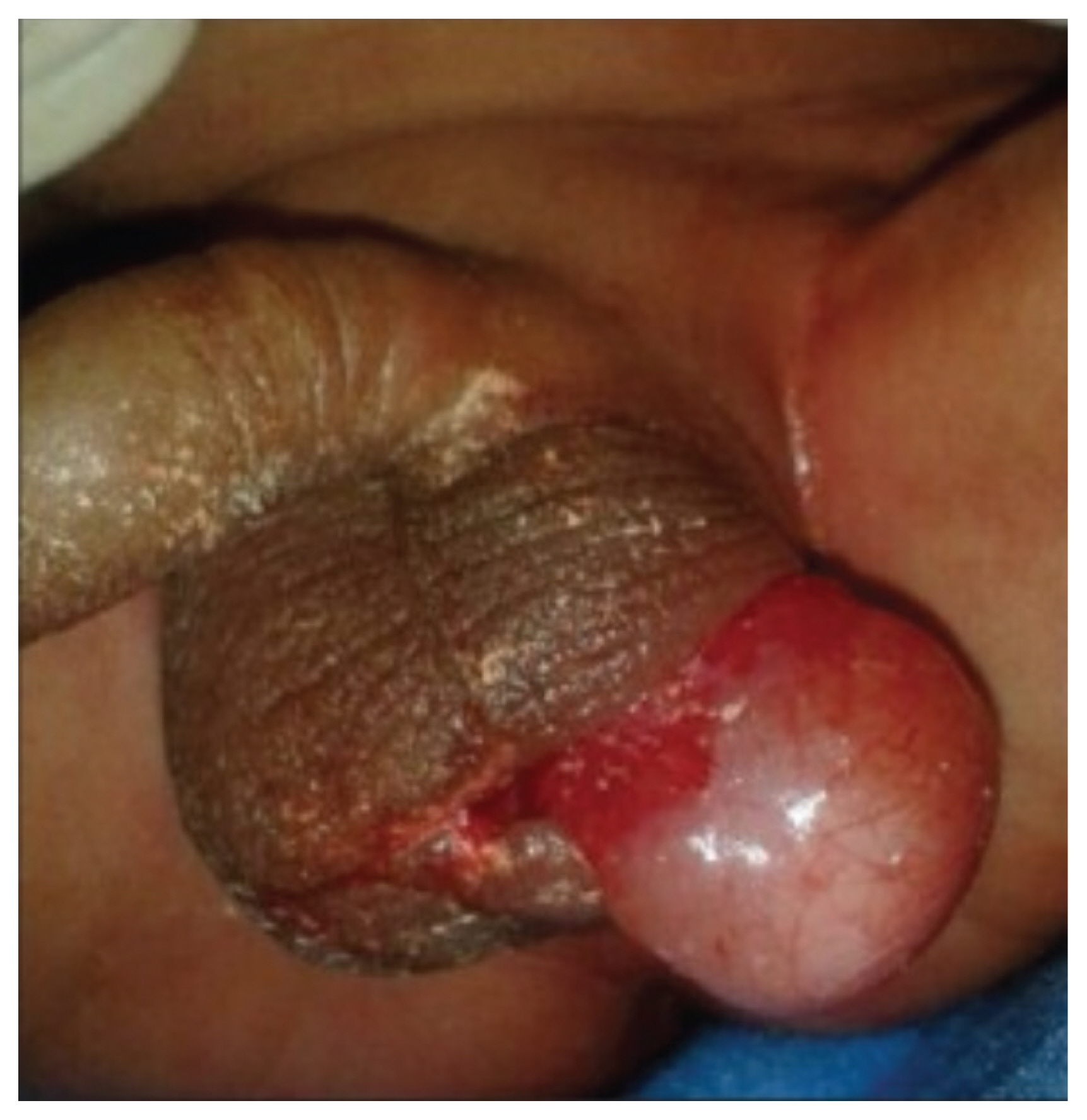
Magnetic resonance imaging at 31 weeks of gestation. Arrowheads indicate a 1.0×1.2 cm mass located posterior to the scrotum corresponding to a perineal lipoma (reprinted with permission from Murase N, Uchida H, Hiramatsu K. Accessory scrotum with perineal lipoma diagnosed prenatally: case report and review of the literature. Nagoya J Med Sci 2015;77:501–6) [38].

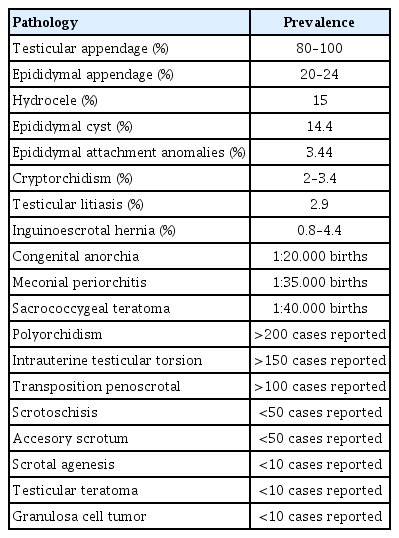
Penoscrotal hypospadias assessed with three-dimensional ultrasonography in rendering mode at 33 weeks of pregnancy, showing a short penis and evidence of testicles inside a bifid scrotum (arrows) (reprinted with permission from Rios LT, Araujo Júnior E, Nardozza LM, Caetano AC, Moron AF, Martins Mda G. Prenatal diagnosis and postnatal ultrasound findings of cloacal anomaly: a case report. Case Rep Pediatr 2012;2012:969860) [59].
5. Scrotal masses
Rhabdomyosarcoma is the most common solid scrotal mass in childhood, which accounts for 75% of all pediatric scrotal cases [49]. Other less frequent tumors include hemangiomas, lipomas, lipoblastomas, and scrotal pearls [18,34,50–52]. Prenatal diagnosis of these entities is practically null because of their small size, being diagnosed in the context of other more obvious anomalies such as an accessory scrotum [53].
Hydrocele, MPO, and inguinoscrotal hernias can also be detected using ultrasound. Hydrocele is the presence of fluid within the scrotum [54]. It is considered the most common finding, with a prevalence of 15% in fetuses [55]. Ultrasound imaging shows a crescent image surrounding the testicles [32]. The vaginal process is permeable throughout pregnancy and a small amount of peritoneal fluid can reach the scrotal sac, leading to scrotal hydrocele. Hence, it is usually believed that hydrocele does not produce significant sequelae [55]. However, there have been no recent studies on this issue. One of the few studies showed that 13.5% of the cases were associated with epididymal abnormalities [56].
MPO is caused by fetal meconium peritonitis, with subsequent passage of the meconium to the scrotum through the permeable vaginal process (Fig. 3) [57]. Its estimated incidence is 1 in 35,000 live births [58]. Normally, it is diagnosed at birth and usually presents as a soft scrotal swelling, hard testicular masses, or hydrocele [10]. Prenatal ultrasound signs for diagnosis include fetal ascites associated with bilateral hydrocele and peritesticular calcification [60–63]. Cystic fibrosis has been observed in up to 15% of the cases. Therefore, screening for this disease is recommended [64]. In asymptomatic masses, conservative management may be adopted while waiting for spontaneous resolution [33]. Surgery is recommended in symptomatic cases or in patients with suspected eoplasm [65].
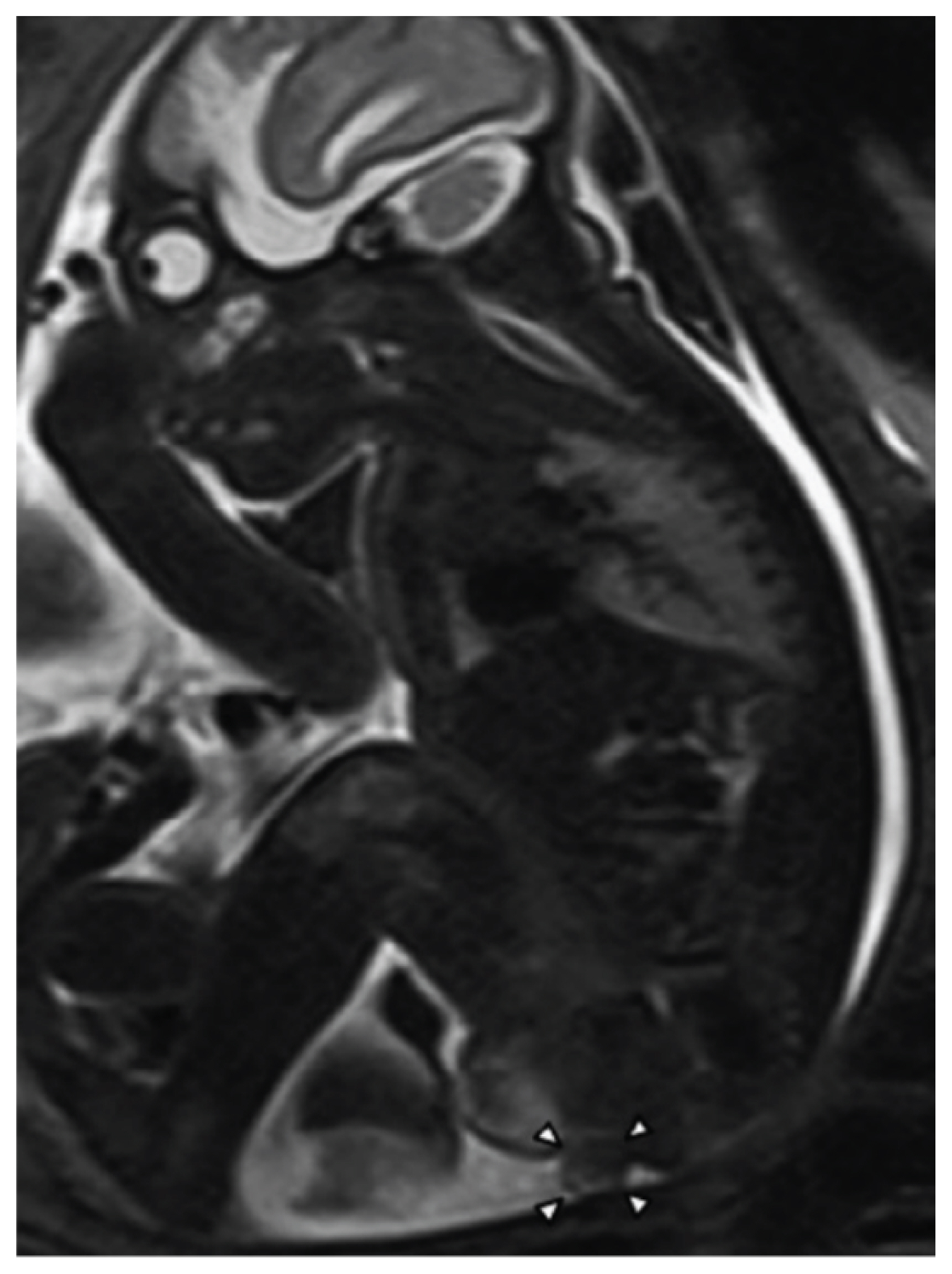
Bilateral hydrocele with peritesticular calcification at 28 weeks of gestation, suggesting a meconium periorchitis (arrows) (reprinted with permission from Ochiai D, Omori S, Ikeda T, Yakubo K, Fukuiya T. A rare case of meconium periorchitis diagnosed in utero. Case Rep Obstet Gynecol 2015;2015:606134) [61].
Inguinoscrotal hernia is a relatively common postnatal finding with an incidence of 1–4%, increasing up to 40% in extremely premature infants (Fig. 4) [66]. Prenatal diagnosis is extremely limited with approximately 20 published cases [9]. The reason for this disparity is the lack of intra-abdominal pressure during pregnancy [67]. One of the most important associated factors is prematurity, with an incidence 20 times greater in infants weighing less than 1,500 g and a high prevalence of incarceration. This is due to the failure of obliteration of the vaginal process that normally occurs in the last weeks of gestation [68,69]. The pathognomonic sign on ultrasound is the presence of intestinal handles in the scrotal sac [9]. Peripheral displacement of the testicles and absence of flow within the mass can also be observed [68,70,71]. The preferred treatment for hernia is surgery, which is performed shortly after the diagnosis due to the risk of incarceration [69].
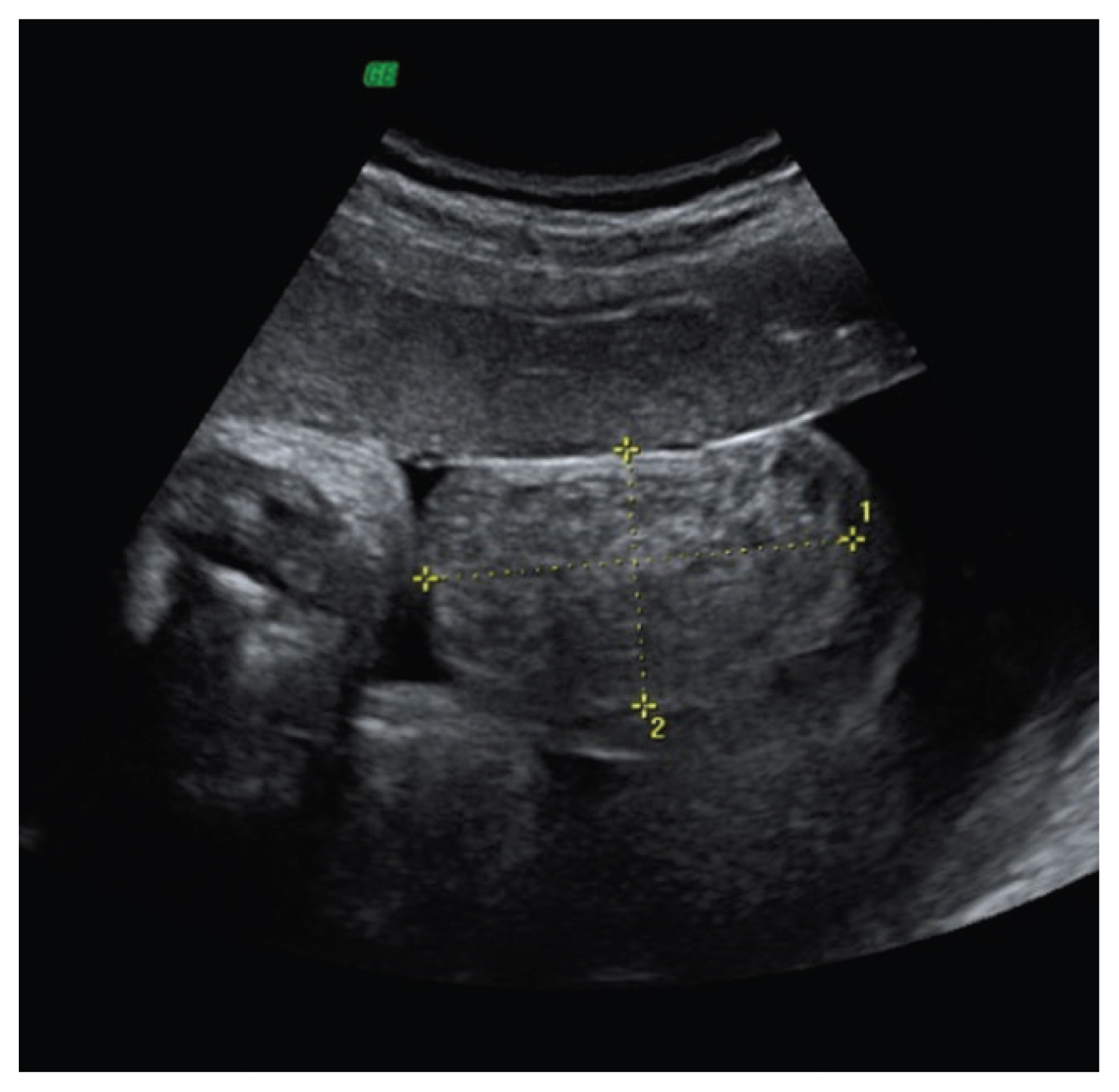
Inguinoscrotal hernia visualized as a scrotal mass (50×46 mm) with mixed echostructure and regular walls, containing few small echo-free cystic areas on the right side (reprinted with permission from Massaro G, Sglavo G, Cavallaro A, Pastore G, Nappi C, Di Carlo C. Ultrasound prenatal diagnosis of inguinal scrotal hernia and contralateral hydrocele. Case Rep Obstet Gynecol 2013;2013:764579) [54].
6. Other entities
Some pathologies with a much lower incidence that deserve a mention include hematocele after adrenal hemorrhage [72], presence of splenic or adrenal ectopic tissue [73–76], and even the scrotal location of a parasitic twin in the entity known as fetus-in-fetu [77].
We would also like to highlight sacrococcygeal teratoma, which is the most frequent extragonadal germ tumor with an incidence of 1 in 40,000 live births [78]. Although it develops at the tip of the sacrum, it can reach a large size extending through the perineum and is often confused with a scrotal mass with a characteristic complex structure [32,70]. Its nature is benign and the risk factors are related more closely to its size and the speed of growth. In extreme cases, it can cause complications such as fetal edema or heart failure that require early delivery and elective cesarean section or even EXIT surgery [79].
1) Testicular pathologies
Cryptorchidism is the main testicular pathology, which is the most common congenital anomaly in male newborns [80]. Other significant pathologies include testicular agenesis, intratesticular masses, and IUTT.
7. Cryptorchidism
Cryptorchidism or undescended testicles involves the failure of the testes to descend permanently in their terminal scrotal position [80]. The estimated incidence at birth is 2–3.4% [7,17,81], although up to one-third of the cases resolve spontaneously during the first 3 months [81]. Seventy-five percent of the cases are usually unilateral and the undescended testes can be palpated in the inguinal canal [82]. Etiology is not clear, although a variety of risk factors such as defects in specific genes, prematurity, low birth weight, intrauterine growth restriction, alcohol consumption, and maternal smoking have been suggested [26,80,81,83–86]. The main complications include reduced fertility, an increased risk of testicular cancer, and the possibility of associated abnormalities. Evidence suggests that both the location and the time of cryptorchidism correlate with the loss of germ cells and Leydig cells [87]. Paternity rates are similar in case of unilateral cryptorchidism, but significantly lower (33–65%) in case of bilateral cryptorchidism [88]. Cryptorchidism increases the risk of testicular cancer by 3 to 8 times [89]. Orchiopexy before adolescence reduces this risk, but it is still more than twice the risk in general population [8]. Associated abnormalities may exhibit a high incidence, with a potential risk of perinatal complications [17,90]. Postnatal management is extremely protocolized and there are several recent clinical guidelines on the subject [8,91]. The main objectives suggested by the Canadian Urological Association (CUA) guidelines include maximizing the possibility of adequate hormonal production and fertility potential, treating associated conditions, and preventing testicular torsion [8]. The CUA guidelines also insist on early detection to determine adequate follow-up and referral for specialized treatment and management. Treatment involves orchiopexy as soon as the patient is clinically stable for anesthesia between 6 and 18 months of age, although the American Urological Association prefers performing it during the first year of life [91]. Orchiectomy is reserved for cases wherein the malignant potential is significant and the testicles cannot contribute to fertility, as observed in the case of post-pubertal cryptorchidism [92].
8. Testicular agenesis
In case of an absent or impalpable testicle in the scrotum, it is necessary to differentiate among a testicle that is present but does not descend (cryptorchidism), a testicle that atrophies and disappears during fetal life (vanishing testis syndrome/ testicular regression syndrome), and an absent testicle that never developed (congenital anorchia) [93,94]. Cryptorchidism is the most common congenital defect, while congenital anorchia is considered a very rare condition observed in approximately 1 in 20,000 live births [95]. In the vanishing testis syndrome, the presence of spermatic cord structures imply the presence of a testicle during early intrauterine life [93]. In addition, a normal morphology of the penis and scrotum indicates testosterone production at least until the 16th week [28]. Several theories have been suggested to explain the absence of a testicle. These include discontinuation of vascular supply, absence of development, or atrophy caused by IUTT [28]. The observation of a true vanishing testicle in the fetal scan could prevent the misdiagnosis of an impalpable testis in the postnatal period and thus, avoid the need for exploratory laparoscopy. However, in 6% of the cases, residual testicular tissue may be observed, which could be associated with an increased risk of malignant degeneration. Hence, some authors advocate the removal of blood vessels as well as the surrounding tissue in these cases [96].
9. Testicular masses
The incidence of prepubertal testicular neoplasms is 5 per 100,000, which is 10 times lower than that in the postpubertal stage and fetal findings are extremely rare [97]. The most frequent histological type is the yolk sac tumor followed by teratoma, although some studies have reported the latter as the most frequent type [18,97,98]. However, the few published cases of prenatal diagnosis involve teratomas and granulosa cell tumors [99–102]. The cause is probably the characteristic sonographic aspect of both the entities. Teratomas appear as large complex cystic masses that are sometimes hypervascularized and show calcifications [103]. Granulosa cell tumors also exhibit a multicystic appearance [101]. In contrast, yolk sac tumors appear as discrete, circumscribed masses in the testicle [104]. Management depends on the benign or malignant behavior of the mass, although partial or total orchiectomy is a frequent treatment approach. In invasive cases, chemotherapy and radiotherapy are considered [100,101].
10. Intrauterine testicular torsion
IUTT was previously included under perinatal torsion that occurred before 30 days of life. Currently, it is distinguished as an entity suspected in utero or in the immediate neonatal examination [105,106]. Due to these changes in the nomenclature, its incidence is not well known. However, at least 150 cases have been published in the literature and 22 of these were bilateral [107]. Etiology of IUTT is unknown. Some theories suggest etiological factors such as high birth weight, the trauma of a complicated birth, or an overactive crematoric reflex. However, the most popular theory suggests that the extreme mobility of the tunica vaginalis within the scrotum allows torsion due to crematoric contractions [108]. These torsions usually occur between the 34th and 38th weeks or during delivery [107]. Ultrasound signs include the absence of Doppler flow on the affected side and the appearance of enlarged and heterogeneous testicles with hypoechoic areas [107,109]. Accumulation of hemorrhagic fluid can also be observed between the layers of the tunica vaginalis, showing a “double ring hemorrhage” appearance, contralateral hydrocele, and deviation of the scrotal septum toward the affected side [105]. However, treatment of prenatal torsion is controversial. Most of the authors have reported that antenatal torsion represents an irreversible intrauterine phenomenon with a salvation rate of less than 5% and termination of pregnancy should be considered urgently after the diagnosis of acute cases [6,110]. Bilateral torsion is considered a true emergency due to the risk of an abnormality, indicating urgent surgery in the newborn [6,111].
11. Other entities
Other notable testicular pathologies include testicular ectopia, scrotoschisis, polyorchidism, and testicular lithiasis (Fig. 5). Testicular ectopia differs from cryptorchidism in that the position of the testicle is outside the line of descent and the superficial inguinal sac is the most common location [112,113]. Extrusion of the testis through a scrotal defect or scrotoschisis can occur due to MPO, in which accumulation within the scrotum reaches a pressure threshold that results in rupture [29]. It can also occur due to a lack of development of the cremaster muscle [112]. Polyorchidism is characterized by the presence of more than two testicles. To date, approximately 200 cases have been reported. Among these, 7 cases exhibited the presence of 4 testicles [112,114,115]. Testicular lithiasis is characterized by the presence of diffuse calcifications [51]. This is a relatively frequent finding with a prevalence of 2.9% in the United States [116]. Its management is under debate, since it is associated with several medical conditions and a 22-fold increase in the incidence of malignant tumors [50,116].
1) Epididymal pathologies
Epididymis is a paratesticular structure that has not been described in prenatal ultrasound despite its important role and a high prevalence of associated abnormalities. We have included the presence of epididymal cysts, anomalies of the testicular connection, and epididymal appendages in this review. Epididymal cysts are common findings with a prevalence of 14.4% in the pediatric population, increasing up to 35.5% in children over 15 years of age [117]. Epididymal cysts contain fluid similar to that observed in the rete testis. Clinically, they are considered benign cysts that do not require further evaluation [51]. Anomalies of the connection between the epididymis and the testis have various classifications. Therefore, the data on their prevalence are variable. Favorito et al. [118] reported a prevalence of 3.44% in normal fetal testes and 36.12% in cryptorchid testes. The association with other anomalies, especially with cryptorchidism, has implications for future fertility as well as for the technical aspects of subsequent surgery [118,120]. Epididymal appendages are found in 20–24% of individuals [121]. Their functions are not well known, although it seems that they can control the amount of serous fluid in the tunica vaginalis space [122]. Torsional appendages account for 50% of the cases of acute scrotum in prepubertal boys [123].
12. Prenatal diagnosis
Currently, the review of the scrotum and internal structures is not established in the first-trimester and second-trimester ultrasound examinations [1,2], mainly because testicular descent does not occur at least until the 24th week [4]. Moreover, they are not usually examined during the third-trimester ultrasound or in the detailed morphological examinations recommended after the diagnosis of most of the fetal abnormalities, resulting in a significant lack of knowledge about diagnosis and management of genital pathologies. This is reflected in the scientific literature predominantly consisting of clinical cases and literature focused on entities with striking ultrasound features such as MPO or penoscrotal transposition [10,36]. Consequently, there is a trend of significant differences between prenatal findings and postnatal prevalence, as observed in the case of benign testicular tumors, cysts, and epididymal appendages or testicular microlithiasis [18,116]; absence of studies about more detailed structures such as the epididymis [124]; scarce and heterogeneous normative data [4,22]; and a lack of management of findings such as hydrocele or undescended testicles [27,55]. IUTT could be the main exception to this trend, with studies focused on its diagnosis and management [6,108,125]. There are several benefits of evaluation of the scrotum and internal structures.
13. Manifestation of genetic syndromes, hormonal disorders, and associated anomalies
Malformations such as bifid scrotum, scrotal agenesis, or cryptorchidism may indicate an undervirilization process, suggesting the presence of a DSD or genetic syndromes with significant morbidity such as the Smith-Lemli-Opitz syndrome or the Prune-Belly syndrome [126,127]. Cryptorchidism, is a known component of almost 400 different syndromes including many with important comorbidities [8]. Similarly, the high rate of associated anomalies in multiple systems, especially in the urinary system, would require a more thorough anatomical review.
14. Diagnosis of subsidiary pathology for immediate intervention
In cases of IUTT at more than 34 gestational weeks, Melcer et al. [6] recommend immediate delivery and surgical intervention in unilateral affection of less than 24 hours of evolution or bilateral affection to save the compromised testicles. A meta-analysis by Monteilh et al. [125] suggested that urgent surgery should be considered if testicular viability is suspected.
15. Diagnosis of subsidiary pathology during postnatal assessment
Although the prenatal findings of pathologies such as inguinoscrotal hernia, MPO, or cryptorchidism cannot be adequately addressed until the postnatal stage, additional information can be obtained that might help reduce the anxiety of the parents, plan the delivery in reference centers, and prepare the medical team [7]. For example, the presence of abnormalities associated with cryptorchidism may allow planning of the surgical approach [69]. It is also possible to predict the need for postnatal surgery, to avoid unnecessary interventions, or to determine long-term fertility [99,118,128].
16. Clinical knowledge of normal variants and pathology
It is unclear how to interpret and act on many of the incidental findings including abnormalities present in a considerable proportion of fetal population such as hydrocele (15%), epididymal cysts (15%), testicular appendages (20–24%), and epididymal appendages (80–100%) [54,56,122]. An increase in the frequency of ultrasound studies would allow formulating the patterns of action and the evidence to support them.
Similarly, evaluation of the male genitals may have unwanted effects. An increase in the findings could lead to a greater number of diagnostic tests and follow-ups, which implies an increase in the expenses as well as in the anxiety of parents and family members [51]. Nevertheless, systematic examination should be included in the third-trimester ultrasound, a test already questioned for its apparent lack of significant benefits to the mother or the baby, for the reasons already discussed in previous sections [129,130]. It could also lead to complications and confusion by interfering with pathologies that are fully protocolized at the postnatal stage. For example, CUA guidelines for cryptorchidism do not recommend the use of imaging tests, as they cannot adequately discern the presence of intra-abdominal gonadal tissue or they might produce iatrogenic effects by confusing undescended testes with retractile testes [8].
Conclusions
The scrotum and internal structures show a wide range of pathologies, with varying degrees of prevalence, morbidity, and mortality. The majority of the reported cases are incidental findings, diagnosed by striking signs on ultrasound examination or detected retrospectively in the postnatal stage. Normative data or data regarding management of these entities are scarce. Active evaluation of these structures could have important benefits in the diagnosis and prenatal management, although there are disadvantages such as an increase in the number of diagnostic tests or parents’ anxiety. Well-designed contemporary studies are necessary to improve our knowledge regarding this subspecialty.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
This study does not require approval of the Institutional Review Board because no patient data is contained in this article. The study was performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
Written informed consent and the use of images from patients are not required for the publication.
Funding information
None.