Is there an association between platelet and blood inflammatory indices and the risk of gestational diabetes mellitus?
Article information
Abstract
Objective
Gestational diabetes mellitus (GDM) is the most prevalent medical complication in pregnancy. Early diagnosis of GDM can influence maternal/neonatal outcomes. To assess the association between platelet and blood inflammatory indices and the risk of GDM occurrence using the complete blood count (CBC) test. We also aimed to determine the sensitivity of each parameter for an early screening of this disorder during pregnancy.
Methods
This case-control study included 2 groups of 110 pregnant women with and without GDM. The women in each group were compared after the routine screening for GDM and after the CBC test at 24–28 weeks' gestation after being matched according to the inclusion criteria. Data were analyzed using SPSS version 16 and Medcalc version 14.8.1 software.
Results
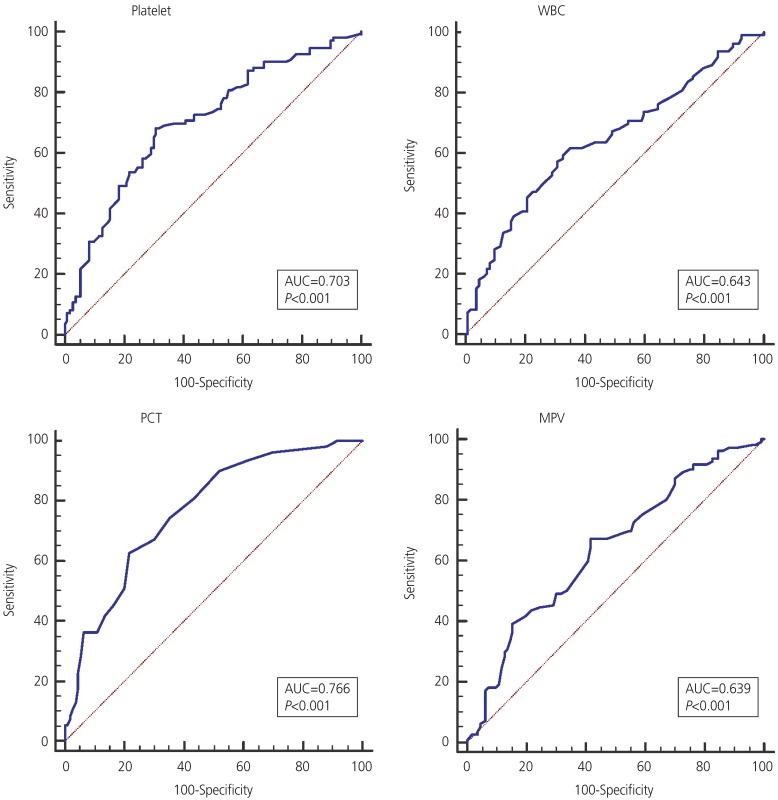
There were statistically significant intergroup differences in white blood cell (WBC) count, platelet count, mean platelet volume (MPV), plateletcrit (PCT), MPV to platelet ratio, platelet to lymphocyte ratio, and Rh values. The values of lymphocyte count, neutrophil count, neutrophil to lymphocyte ratio, and blood group were not significantly different between groups. The logistic regression analysis showed the predictive values of WBC, platelet, MPV, and PCT in GDM. According to the receiver operating characteristic curve for all 3 variables, the level below the PCT chart was more than that of the others.
Conclusion
Increasing platelet and inflammatory indices on the CBC test in the second trimester of pregnancy seemed to be associated with the probability of GDM occurrence.
Introduction
Gestational diabetes mellitus (GDM) is among the most common chronic diseases during pregnancy; in fact, it was recognized by one study as the most common medical condition in pregnancy [1]. GDM predominantly occurs during pregnancy in women whose pancreatic function is insufficient to overcome the insulin resistance caused by pregnancy [2]. Despite more than 50 years of research, no general agreement on the screening method of GDM has been achieved [1]. The worldwide estimated prevalence of GDM is 13.2% [3]. The prevalence of GDM in the most recent Iranian study was reported as 81.8% [4]. Also, the rate of GDM relapse is reportedly 30–69% [5]. Early screening for the detection of GDM can prevent maternal/fetal/neonatal outcomes such as preeclampsia, cesarean section, polyhydramnios, fetal macrosomia, instrumental labor, and prenatal mortality [678]. According to International Diabetes Federation guidelines, the diagnosis of GDM occurs when a glucose tolerance test performed at 24–28 weeks of pregnancy reveals a fasting blood glucose greater than 92, glucose level 1 hour after ingesting 75 g of glucose of up to 180 mg/dL, or a glucose level 2 hours after ingesting 75 g of glucose greater than 153 mg/dL [9]. The pathophysiological process of diabetes occurs weeks to months before the diagnosis, and factors associated with the disease can be present in the blood before the clinical diagnosis of GDM is made [10]. A high level of placental lactogen hormone is associated with increased lipolysis, and the release of free fatty acids may increase insulin resistance [1]. On the other hand, chronic and mild inflammation are considered key components of the pathogenesis of insulin resistance and type 2 diabetes [11].
Among immunity elements, neutrophils, lymphocytes, and platelets play an important role in controlling inflammation. An increased count of leukocytes, especially neutrophils, is seen in both chronic and acute inflammation [1213]. The platelet to lymphocyte ratio (PLR) and neutrophil to lymphocyte ratio (NLR) as inflammatory indices can be easily and inexpensively measured using this test [14]. Platelet indices including platelet count, mean platelet volume (MPV), platelet distribution width (PDW), plateletcrit (PCT), and mean platelet volume to platelet (MPR) ratio are derived from the results of a complete blood count (CBC) [15]. CBC and its differential count are inexpensive and convenient tests that can provide important information for diagnosing disease [16]. Various studies have suggested changes in some indicators including blood cells and platelets in GDM. In a meta-analysis study of 191 observational studies consisting of 1,361 women with GDM and 1,911 non-diabetic women, MPV values increased in the GDM group [17]. On the other hand, a cross-sectional study in Guangzhou, China [18], showed that in 302 pregnant women with GDM and 310 non-diabetic women, significant increases in white blood cells (WBCs), red blood cells, and plaques were significantly higher in pregnant women with GDM than in those without GDM. However, in a cross-sectional study of 20,128 people in Nangbo, China, no statistically significant difference between platelet and PDW levels, in diabetic and non-diabetic groups was reported (P>0.05) [19].
Given the contradictory results of these indices and a lack of Iranian studies, this study aimed to investigate the association between platelet and blood inflammatory indices and the risk of GDM occurrence using maternal CBC sampling in the second trimester of pregnancy.
Materials and methods
This case-control study was conducted from January to October 2018 and included 2 groups consisting of 220 healthy pregnant women (n=110) and women with GDM (n=110) referred to the midwifery clinic of Shohada-e-Tajrish Hospital, Tehran, Iran. The minimum sample size for each group was calculated using the following formula [2021]:

At α: 0/05 and 80% power.
Written informed consent was provided by all participants. Demographic data including age, gestational weeks, education level, and occupations of the mother and father were collected via interview. Pre-pregnancy weights and blood types were collected from their medical files. Each subject's height was measured by the researcher and the body mass index (BMI) was calculated. Also, due to the effect of increasing the number of pregnancies on the risk of GDM, sampling was performed only from primiparous mothers and mothers with a second pregnancy who had healthy children. Sampling in both groups was performed using the convenience method from pregnant women referred to the midwifery clinic of Shohada-e-Tajrish Educational Hospital in Tehran, Iran.
The 2-hour oral glucose tolerance test with 75 g of oral glucose and CBC tests were performed at weeks 24–28; if GDM was confirmed by a gynecologist based on the national GDM screening guideline, the women were continuously recruited using those criteria.
The inclusion criteria were as follows: gestational age 24–28 weeks based on last menstrual period or ultrasound in the first trimester of pregnancy, BMI of 18.5–29.9 before pregnancy, no history of GDM no overt diabetes, no family history of type 2 diabetes, singleton spontaneous pregnancy, no hookah use or cigarette smoking, no drug or alcohol use, no medication use other than supplements, no previous/current infectious disease, and no medical or obstetric disorders in the current pregnancy (e.g., cardiovascular disease, thyroid diseases, polycystic ovary syndrome). Unwillingness to cooperate with this study or a diagnosis other than GDM were the exclusion criteria. Next, using the group matching method, sampling for the control group was performed from women at low risk of developing GDM. For the group matching, an eligible pregnant woman was first entered into the case group; then, using the individual matching method, a subject of the same age, parity, and BMI was selected for the control group. The procedure was repeated for all cases included in the study until the desired study sample was achieved.
Blood samples were taken from pregnant women at Shohada-e-Tajrish Hospital at 24–28 weeks' gestation. Platelet and inflammatory parameters in the CBC were compared between groups. The correlations between those values and the incidence of GDM was compared and analyzed.
1. Screening for gestational diabetes mellitus
GDM screening was performed at 24–28 weeks of gestation for all pregnant women. A fasting blood sample was taken from all pregnant women for glucose monitoring. It was repeated 1 and 2 hours after the ingestion of 75 g of glucose. The normal fasting blood glucose should be less than 92 mg/dL, the level at 1 hour later should be less than 180 mg/dL, and that at 2 hours later should be less than 153 mg/dL. If any of the levels exceeded these limits, GDM was diagnosed.
2. Laboratory analyses
Blood samples were taken for the analysis of CBC and GTT values in the morning after an 8-hour fast. For this purpose, 5 mL of intravenous blood was collected from the antecubital vein with minimal trauma into a tube containing ethylenediaminetetraacetic acid. Blood samples were analyzed within 1 hour after collection. For this purpose, blood serum samples were separated in tubes using a centrifuge at 3,000×g for 10 minutes. The specimens were then placed in a Sysmax K-1000 Hemato Analyzer tube (Sysmex Corporation, Kobe, Japan) for the CBC. Serum glucose levels were assessed using spectrophotometry. A Hitachi 912 Auto-Analyzer (Hitachi, Tokyo, Japan) and Bionik Glucose Kit (Bionik, Tehran, Iran) were used to measure blood glucose.
Instrument reliability was verified using the calibration method and blood glucose kit daily, 3 times in the morning, evening, and night shifts, using special calibration solutions.
3. Statistical analysis
SPSS software version 16 (IBM Inc., Chicago, IL, USA) was used to analyze the data using the 2-independent samples t-test, Mann-Whitney U test, and logistic regression tests. MedCalc software version 14.8.1 (MedCalc Software, Mariakerke, Belgium) was used to assess the cutoff points in GDM-dependent variables and determine their sensitivities and specificities.
Results
Given the matching of the most important demographic and medical variables in the groups, no statistically significant intergroup differences were noted (Table 1). The majority of women (43.6%) in both groups had a secondary education level. Most were housewives (79%) with self-employed husbands (35.4%). The laboratory findings showed no significant intergroup differences in lymphocyte counts, neutrophil counts, NLR, and blood group variables (Table 2).
WBC, platelet, MPV, PCT, MPR, PLR, and Rh variables were significantly higher in the GDM group than in the control group. Also, more women with GDM were Rh-positive. To control for the simultaneous effects of other variables on GDM using logistic regression, increasing values of PCT, WBC, platelet, and MPV were significantly correlated with GDM (Table 3). The receiver operating characteristic (ROC) chart showed that in increase of 0.2 points in PCT was the most closely related, with a sensitivity, specificity, positive predictive value, and negative predictive value of 67.3, 70, 69.1, and 68.1, respectively (Table 4). PCT had the highest area under the curve (0.766) for predicting GDM (Fig. 1).

Association between platelet and inflammatory indices and gestational diabetes mellitus using logistic regression analysis
Discussion
Our study results suggested that PCT value in the second trimester had the highest correlation with the occurrence of GDM. However, based on the contractual classification system, the surface below the ROC curve in the range of 70–80 was not a strong predictor of clinical sensitivity or specificity [22]. Since there is no a practical way to predict maternal GDM before screening tests, the early detection of inflammatory and platelet-enhancing indicators using the CBC test in the first half of pregnancy can help the assessment of maternal health. This can remind healthcare providers of neonatal and maternal health and prevent inappropriate outcomes.
Platelet indicators, which are platelet activation biomarkers, allow the performance of a wide range of clinical studies with various diagnostic values and prognoses without additional cost. Among the PCT, MPV, and PDW platelet indices, a group of platelet parameters are reported in the CBC assay [15]. Besides the known platelet functions of homeostasis and thrombosis, a large number of studies have shown that platelets play an important role in intercellular communication, inflammatory activity, and immunization [23]. The results of studies by Gorar et al. [21], Sahbaz et al. [24],and Çeltik et al. [25] showed that women with GDM had significantly higher values of platelet and MPV than healthy pregnant women in the second trimester of pregnancy, which was similar to the results of the present study. However, Erdoğan et al. [26] found no association between MPV and GDM. The reason for this result was the low number of samples compared to the other 3 studies.
PCT, as the product of platelet and MPV, provides complete information about the total platelet mass and is clinically more valuable than either platelet or MPV [2427]. The findings of studies by Sahbaz et al. [24] and Erdoğan et al. [26] showed that pregnant women with GDM had significantly higher PCT levels than healthy pregnant women, which was consistent with the results of this study. Moreover, in the Sahbaz et al.'s study [24], PCT was the most powerful predictor of GDM in the second semester.
MPR, a new indicator for predicting inflammation, is a sign of a bad outcome [2829]. The results of the study conducted by Duman et al. [28] showed that MPR levels were significantly higher in people with type 2 diabetes than in healthy individuals. This finding was consistent with the results of our study.
Pattanathaiyanon et al. [30] showed that higher numbers of leukocytes at the beginning of pregnancy were associated with a greater risk of developing GDM. The result of the study by Sargın et al. [14] suggested that WBC, neutrophil, and lymphocyte parameters in pregnant women with GDM were higher than those in healthy pregnant women. In the present study, only WBC was significantly correlated with GDM, and the findings regarding lymphocytes and neutrophils were in agreement with those of the Sargın et al.'s study [14]. Gorar et al. [21] reported that none of the 3 variables were significantly correlated with GDM.
On the other hand, inflammation has been reported in the pathogenesis of GDM. For example, Gomes et al. [31] and Catalano [32] showed that 2 inflammatory cytokines in GDM, specifically interleukin-6 and tumor necrosis factor-α, were increased, while that of interleukin-10 was decreased. NLR and PLR have been introduced as inflammatory factors, and their associations with cardiovascular, pulmonary and other malignancies have been reported [33343536]. The findings of the Sahbaz et al. [24] study suggested a significant correlation between increased levels in women with GDM compared with healthy pregnant women. In a study, only PLR had a significant correlation with GDM. In the Sargın et al. [14] study, no NLR or PLR variables were associated with the incidence of GDM.
Saadati et al. [37] reported that the blood types did not differ significantly between groups, consistent with the results of our research. Most women with GDM have blood type A, but in this study, women with blood type O were more commonly affected. Shimodaira et al. [38] reported a correlation between blood type and GDM. In women with blood type AB, the risk of developing GDM was reported to be higher, which contradicted with the results of the p resent study. The findings of the study by Huidobro et al. [39] showed no correlation between maternal blood sugar and GDM, which was in contrast to the results of this study. Such a difference in results seems due to differences in the research environment such as Iran, Japan, and Chile, as well as sex differences. In the present study, according to the distribution of Rh in the Iranian population, a positive Rh status was much more abundant than a negative Rh status. In addition, the difference between the positive and negative groups was negligible. As a result, this index has no significant relationship with the incidence of GDM.
One of the limitations of this study was its cross-sectional design, although the case-control design and group matching aids the analysis and confirmation of the correlations between the study variables. Other medical and obstetric variables were reported based on their midwifery files and through interviews. Future studies using the cohort design (a futuristic cohort) of changes in inflammatory and platelet factors in the first trimester and follow-up to the end of pregnancy are suggested.
In conclusion, the findings of this study confirmed the presence of a direct and significant correlation between platelet and inflammatory indices in the CBC test in the second trimester of pregnancy and GDM. WBC, platelet, MPV, PCT, MPR, and PLR values were higher in women with GDM than in healthy pregnant women. PCT had the strongest association with the occurrence of GDM in the second semester, which had special clinical significance. Given the importance of an early diagnosis of GDM, the need for similar studies in the first trimester of pregnancy can improve pregnancy outcomes using early intervention.
Acknowledgments
The author appreciates the collaboration of the research deputy of Shahid Beheshti University of Medical Sciences, Shohda-e-Tajrish Hospital staff, and all the pregnant women who participated in this study.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
Ethical approval: This article was based on a master's degree thesis under the ethics code of IR.SBMU.PHNM.1396.755. The study was approved by the Institutional Review Board of School of Pharmacy and Nursing and Midwifery-Shahid Beheshti University of Medical Sciences (IRB No. IR.SBMU.PHNM.1396.755) and performed in accordance with the principles of the Declaration of Helsinki.
Patient consent: The patients provided written informed consent for the publication and the use of their images.