Is preeclampsia itself a risk factor for the development of metabolic syndrome after delivery?
Article information
Abstract
Objective
This study aimed to determine the association between preeclampsia and the postpartum development of metabolic syndrome based on the pre-pregnancy status.
Methods
Korean women who delivered their first child between January 1, 2011, and December 31, 2012, were enrolled. All subjects underwent a national health screening examination conducted by the National Health Insurance Corporation 1 or 2 years prior to their first delivery and within 2 years after their first delivery.
Results
Among the 49,065 participants, preeclampsia developed in 3,391 participants (6.9%). The prevalence of metabolic syndrome was higher postpartum in women with preeclampsia than in those without preeclampsia (4.9% vs. 2.7%, respectively, P<0.001). Through the pre-pregnancy to postpartum period, women with preeclampsia had a greater increase in gestational weight retention, body mass index, waist circumference, systolic blood pressure, and triglyceride levels and a greater decrease in high-density lipoprotein cholesterol levels than women without preeclampsia. Preeclampsia was associated with an increased risk of the postpartum development of metabolic syndrome in women without pre-pregnancy metabolic syndrome (odds ratio, 1.28; 95% confidence interval, 1.05–1.56). However, preeclampsia was not associated with postpartum metabolic syndrome in women with pre-pregnancy metabolic syndrome or 2 components of metabolic syndrome.
Conclusion
In this study, preeclampsia was associated with the postpartum development of metabolic syndrome in women without pre-pregnancy metabolic syndrome. However, the effects were attenuated by predisposing risk factors in the pre-pregnancy period.
Introduction
Preeclampsia is a pregnancy complication characterized by hypertension (HTN) and proteinuria after 20 weeks' gestation and is seen in 2–8% of pregnancies [1]. Complications of preeclampsia include prematurity, intrauterine growth restriction, and placental abruption [23]. Although high blood pressure (BP) typically normalizes after delivery, women with preeclampsia are at increased risk for eventually developing HTN [45]. These women are at higher risk of future diabetes mellitus (DM), stroke, and cardiovascular disease (CVD) as well as increased overall mortality rates [4567]. However, it remains unclear whether the development of preeclampsia itself predisposes women to long-term CVD or simply arises in women with an underlying susceptibility to future CVD [2]. It is generally believed that preeclampsia does not predispose a woman to CVD; rather, preeclampsia and CVD share common risk factors [8].
Metabolic syndrome, an assembly of metabolic disorders, is associated with an increased risk of the above conditions [91011]. A recent study found that preeclampsia is associated with an increased risk of postpartum metabolic syndrome [12131415], implying its role in the pathophysiology that ultimately leads to CVD [13]. However, none of these studies included pre-pregnancy metabolic assessments; therefore, the presence of metabolic syndrome before pregnancy remains uncertain.
Thus, the aim of this study was to investigate the association between preeclampsia and the postpartum development of metabolic syndrome based on the pre-pregnancy status.
Materials and methods
1. Healthcare delivery system in South Korea
The study was conducted using data from the Korea National Health Insurance (KNHI) Claims Database from 2009 to 2014. Of the total Korean population, 97% is obligated to enroll in the KNHI program, while the remaining 3% of Korean population is covered by the Medical Aid Program. Thus, the KNHI Claims Database contains information on all claims for diseases and procedures covered by the national health insurance. The KNHI healthcare system also includes a biannual national health screening examination (NHSE) provided to all insurance subscribers and dependents.
2. Study population
Among all women in Korea who delivered their first baby between January 1, 2011, and December 31, 2012, those who underwent an NHSE 1–2 years before their first delivery and within 2 years after their first delivery were included. The time before delivery was defined as the time from the pre-pregnancy measurement to the first delivery. The time after delivery was defined as the time from the first delivery to the postpartum measurement. Gestational weight retention was determined by subtracting the woman's weight at a pre-pregnancy visit from her weight at a postpartum visit. Preeclampsia was identified by the International Classification of Diseases-10th revision (ICD-10 codes O12, O13, O14, and O15).
3. Measurement of pre-pregnancy and postpartum characteristics
Data from the NHSE consisting of a health interview and a health examination were used to evaluate pre-pregnancy and postpartum factors of the study population. Information on the participants' age and smoking status, covariates in the study, were obtained from the health interview. Participants were divided into 3 categories according to smoking status: current, past, and never. As employee health insurance premiums reflect a worker's salary, the study used premiums as a substitute variable for income level. Pre-pregnancy insurance premiums were classified into 5 quintiles of performance, with the highest income level in Q5.
During the health examination, each participant's body mass index (BMI, kg/m2), waist circumference (WC) measured at the approximate midpoint between the lower margin of the last palpable rib and the top of the iliac crest, and BP were measured. BP was measured with a standard mercury sphygmomanometer. All participants were required to fast a minimum of 8 hours before the blood samples were obtained. The levels of fasting blood sugar, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured using enzymatic methods. Women with a BP ≥140/90 mmHg at the NHSE (n=152) without a diagnosis of HTN were excluded, as were those with previously diagnosed HTN (n=123).
4. Definition of metabolic syndrome
The National Cholesterol Education Program Adult Treatment Panel III Criteria for metabolic syndrome [11] except those for abdominal obesity were used in this study. Cutoffs established for Korean adults as proposed by the Korean Society for the Study of Obesity [16] were used to evaluate abdominal obesity. The American Diabetes Association guideline was used to evaluate high blood glucose levels [17].
Any 3 or more of the following traits in the same individual met the criteria for metabolic syndrome: 1) high WC, ≥85 cm; 2) high BP, ≥130/85 mmHg or currently on antihypertensive medication; 3) high fasting glucose level, ≥100 mg/dL or currently being treated for DM; 4) low HDL-C, <50 mg/dL; and 5) high TG, ≥150 mg/dL.
5. Statistical analysis
Continuous and categorical variables are expressed as mean±standard deviation and percentages, respectively. Among the groups, the t-test was used to compare the differences in continuous variables, while the χ2 test was used to examine categorical variables. Analysis of covariance was used to compare changes in anthropometric characteristics and components of metabolic syndrome across the pre-pregnancy and postpartum periods after the adjustment for covariates including age, pre-pregnancy BMI, smoking status, insurance premiums, and time before pregnancy and after delivery. Using multivariate logistic regression analysis, we calculated the adjusted odds ratio (OR) and 95% confidence interval (CI) for the postoperative development of metabolic syndrome. We performed the statistical analysis using SAS for Windows, version 9.4 (SAS Inc., Cary, NC, USA).
Results
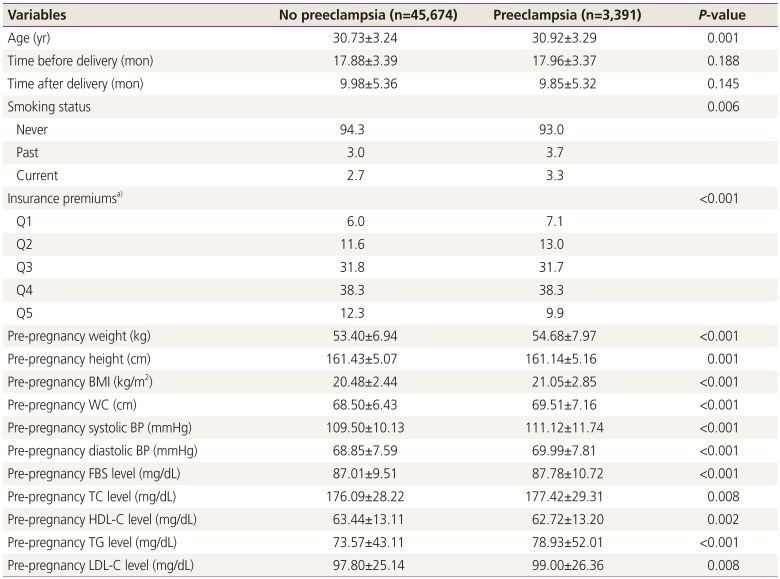
Among the 49,065 participants, preeclampsia developed in 3,391 (6.9%). Table 1 represents the pre-pregnancy characteristics of women who were diagnosed with preeclampsia in their first pregnancy. Women with preeclampsia tended to be older and more likely to have a family history of HTN than those without preeclampsia. The pre-pregnancy BMI, WC, and BP as well as fasting glucose, TC, TG, and LDL-C levels were higher and HDL-C levels were lower in women with preeclampsia than in those without preeclampsia. The distributions of smoking status and insurance premiums differed between the 2 groups. However, time before pregnancy and after delivery did not differ between the 2 groups.
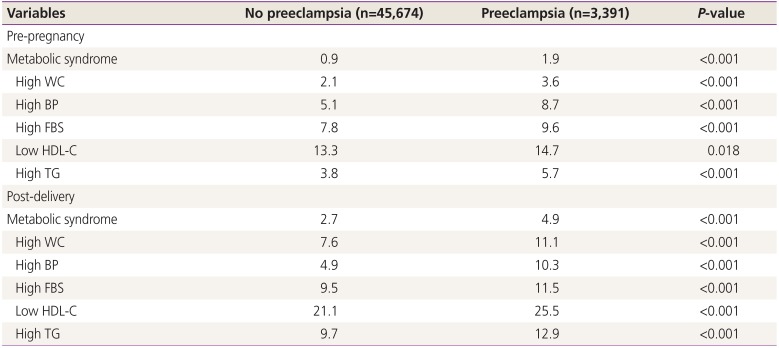
Women with preeclampsia were more likely to show abnormalities in the components of metabolic syndrome and a subsequently higher prevalence of metabolic syndrome before pregnancy than women without preeclampsia (Table 2). The postpartum prevalences of metabolic syndrome and component abnormalities were also higher in women who developed preeclampsia (Table 2).

The prevalence of metabolic syndrome and abnormalities of its components according to presence or absence of preeclampsia
Table 3 shows the changes in the anthropometric characteristics and components of metabolic syndrome through the pre-pregnancy to postpartum periods. Women with preeclampsia had a greater increase in gestational weight retention, BMI, WC, systolic BP, and TG levels and a greater decrease in HDL-C levels than women without preeclampsia. However, there were no intergroup differences in changes in diastolic BP and fasting glucose, TC, or LDL-C levels.
Table 4 shows the risk of developing postpartum metabolic syndrome. Among all women, those with preeclampsia were more likely to develop postpartum metabolic syndrome (OR, 1.34; 95% CI, 1.11–1.61) than those without preeclampsia after the adjustment for confounding factors. Among women without pre-pregnancy metabolic syndrome, preeclampsia was associated with an increased risk of the development of metabolic syndrome (OR, 1.28; 95% CI, 1.05–1.56). However, among women who had metabolic syndrome before pregnancy, preeclampsia was not associated with the postpartum development of metabolic syndrome (OR, 1.62; 95% CI, 0.92–2.85).

The risk of developing metabolic syndrome after delivery according to absence and presence of metabolic syndrome in the pre-pregnancy period
The risk of developing metabolic syndrome postpartum according to the number of components of metabolic syndrome in the pre-pregnancy period is shown in Table 5. Preeclampsia was associated with an increased risk of the postpartum development of metabolic syndrome in women with 0 or 1 components of pre-pregnancy metabolic syndrome, but there was no association in women with 2 or more components.

The riska) of developing metabolic syndrome after delivery for preeclampsia according to number of components of metabolic syndrome in the pre-pregnancy period
Among women with preeclampsia, 81.8% (131/165) of those who developed postpartum metabolic syndrome did not have pre-pregnancy metabolic syndrome. In particular, 31.5% (52/165) did not have any components of metabolic syndrome. In contrast, 18.2% of women who developed postpartum metabolic syndrome already had metabolic syndrome in the pre-pregnancy period, and 68.5% of these had 1 or more components of metabolic syndrome.
Discussion
Here we evaluated the association between preeclampsia and the postpartum development of metabolic syndrome based on the pre-pregnancy status. Among women without pre-pregnancy metabolic syndrome, preeclampsia was associated with an increased risk of the postpartum development of metabolic syndrome. Even in women without any elements of metabolic syndrome in the pre-pregnancy period, preeclampsia was associated with an increased risk of development of metabolic syndrome in the postpartum period.
The potential mechanisms that may link preeclampsia with the postpartum development of metabolic syndrome are not well understood. Pregnancy is associated with weight gain [18], particularly the accumulation and redistribution of body fat [19], which is related to insulin resistance, CVD, and DM [2021]. Moreover, physiological adaptations that occur during pregnancy result in insulin resistance [22] and atherogenic dyslipidemia as well as increases in cholesterol and triglyceride levels [2324]. In contrast, in preeclampsia, the physiological changes in weight, insulin resistance, and lipids are exaggerated [25262728] and can lead to metabolic disturbances. In fact, although women with preeclampsia already had abnormal levels of the components of metabolic syndrome in the pre-pregnancy period, exaggerated changes in the components were found in women with preeclampsia versus women without preeclampsia. This suggests that preeclampsia itself may have an independent effect on the development of metabolic syndrome in the postpartum period through perturbations in multiple metabolic pathways. In contrast, among women who already had pre-pregnancy metabolic syndrome, preeclampsia was not an additional risk factor for the postpartum development of metabolic syndrome. These results suggest that the contribution of preeclampsia to the development of metabolic syndrome is attenuated by the contribution of factors that were present before pregnancy [29].
In this study, women affected by preeclampsia were more likely to develop metabolic syndrome as reported in other studies [12131415]. However, it remains unclear whether preeclampsia itself potentiates the postpartum development of metabolic syndrome or preeclampsia arises in women with predisposing conditions for metabolic syndrome. When the link between preeclampsia and the postpartum development of metabolic syndrome was evaluated only among women with preeclampsia, 31.5% did not have any of the components of metabolic syndrome in the pre-pregnancy period. This also means that 68.5% already had 1 or more components. Therefore, it is likely that the underlying association between preeclampsia and the development of metabolic syndrome is a combination of both possibilities [30].
Based on our results, a structured assessment may lower the risk of metabolic syndrome related to preeclampsia. First, the early identification and intervention, such as lifestyle modifications, may be suggested for women at high risk of developing preeclampsia to lower their risk. It is possible for women with preeclampsia but with no metabolic abnormalities to develop metabolic syndrome in the postpartum period. Thus, preeclampsia should be recognized as a major risk factor for the development of metabolic syndrome, and early interventions should be provided with the goal of primary prevention [31].
There are several limitations in the interpretations of these findings. First, preeclampsia severity, gestational age at delivery, and neonatal outcome, which are known to be associated with the development of CVD, were not reflected [63233]. Data about postpartum lifestyle, such as breast-feeding or physical activity, both of which effectively reduce cardiovascular risk [3435], were also not available. Therefore, further studies are needed to evaluate the differences in these potentially confounding factors between women with and those without preeclampsia. Second, in this study, since pre-pregnancy metabolic syndrome was evaluated, it is possible that women without pre-pregnancy metabolic syndrome will develop metabolic syndrome early in pregnancy; thereafter, preeclampsia is first diagnosed in the absence of a diagnosis of metabolic syndrome, although we attempted to correct these problems by adjusting for risk factors such pre-pregnancy BMI and the time before delivery. However, there is no definition of metabolic syndrome specific to pregnancy. Moreover, AC, a component of metabolic syndrome, will increase significantly as gestational weeks increase. Thus, it is difficult to diagnose metabolic syndrome during pregnancy. Finally, the diagnosis of preeclampsia was based on ICD-10 codes from the KNHI Claims Database. Thus, the validity of the diagnosis is another limitation. There are no formal validation studies of the diagnoses of preeclampsia in the KNHI Claims Database of Korea. However, the KNHI Claims Database includes the entire Korean population; thus, it is the most reliable data for assessing healthcare utilization of the Korean population [36]. According to a previous validation study that compared diagnoses from the database to the actual diagnoses documented in the patients' medical records, KNHI Claims Data had an 83.4% overall positive predictive value in the cases of hospitalized patients [3738]. The diagnostic codes were also more accurate for claims involving conditions with higher severity, such as preeclampsia [3940].
Nevertheless, the strength of the present study lies in its large population-based cohort in which metabolic syndrome was assessed both before pregnancy and after delivery. Moreover, to minimize the effect of parity, we enrolled only women who had delivered their first child during the study period. Our robust metabolic assessments provide important information about the metabolic changes that occur from pre-pregnancy to the postpartum period according to the presence of preeclampsia.
In conclusion, our study demonstrated that preeclampsia was associated with an increased risk of the postpartum development of metabolic syndrome in women without pre-pregnancy metabolic syndrome, but this finding was attenuated by predisposing risk factors for metabolic syndrome in the pre-pregnancy period. Further studies with long-term follow-up periods are needed to compare the development of future CVD between preeclamptic women with newly developed metabolic syndrome and those with pre-pregnancy metabolic syndrome or predisposing risk factors.
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2012R1A1A1044719).
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
Ethical approval: The Institutional Review Board of Korea University Medical Center (IRB No.2016GR0093) reviewed and approved this study.