|
 |
- Search
| Obstet Gynecol Sci > Epub ahead of print |
Abstract
Objective
We aimed to predict the risk of postoperative adjuvant therapy using preoperative variables in young patients with early stage cervical cancer. The predicted risk can guide whether ovarian transposition should be performed during surgery.
Methods
In total, 886 patients with stage IB1-IIA cervical cancer aged 20-45 years who underwent modified radical or radical hysterectomy between January 2000 and December 2008 were included. Preoperative variables, preoperative laboratory findings, International Federation of Gynaecology and Obstetrics stage, tumor size, and pathological variables were collected. Patients with high risk factors or those who met the Sedlis criteria were considered adjuvant therapy risk (+); others were considered adjuvant therapy risk (−). A decision-tree model using preoperative variables was constructed to predict the risk of adjuvant therapy.
Results
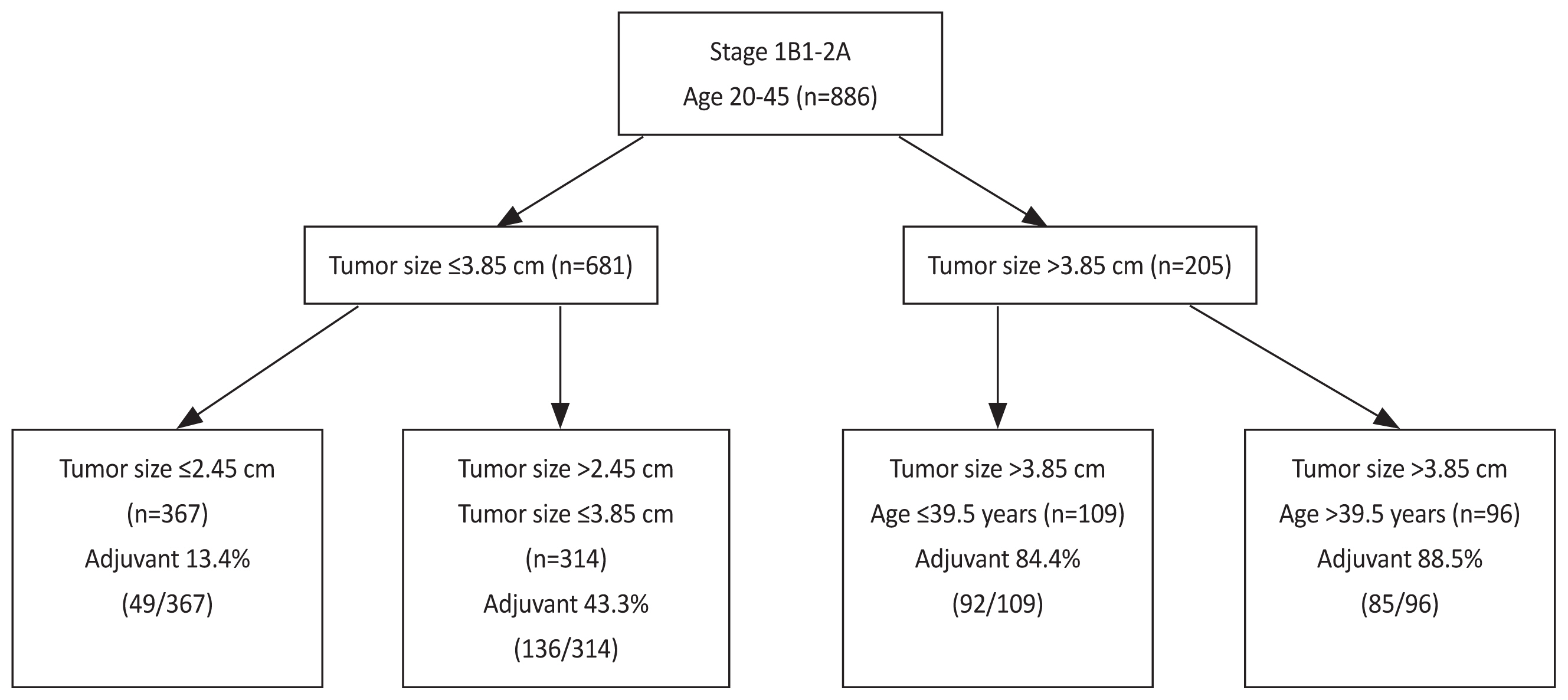
Of 886 patients, 362 were adjuvant therapy risk (+) (40.9%). The decision-tree model with four distinct adjuvant therapy risks using tumor size and age were generated. Specifically, patients with tumor size ≤2.45 cm had low risk (49/367; 13.4%), those with tumor size ≤3.85 cm and >2.45 cm had moderate risk (136/314; 43.3%), those with tumor size >3.85 cm and age ≤39.5 years had high risk (92/109; 84.4%), and those with tumor size >3.85 cm and age >39.5 years had the highest risk (85/96; 88.5%).
Cervical cancer is the fourth most prevalent malignancy in women worldwide, and it remains a major global health care issue [1,2]. The incidence of cervical cancer has decreased since the introduction of a screening programs [3]; however, the incidence in number of young patients is growing [4]. Since 1993, the incidence and mortality rates of cervical cancer among patients <30 years have grown in Korea [5].
The primary treatment for patients with early cervical cancer is surgery with or without adjuvant therapy based on pathological findings of the surgery specimen. Adjuvant therapy consists of pelvic radiation with or without concurrent chemotherapy. Specifically, adjuvant therapy is recommended in patients with high risk factors including positive lymph node (LN), positive surgical resection margins (RM), and involvement of parametrium (PM) or intermediate risk factors such as a large tumor size, deep stromal invasion (DSI), and lymphovascular space invasion (LVSI), commonly referred to as Sedlis criteria [6-8]. No adjuvant therapy is indicated in patients without risk factors. Pelvic radiation with or without concurrent chemotherapy is used as an adjuvant therapy.
Pelvic radiation leads to premature ovarian failure in young patients with cervical cancer [9]. Therefore, the National Comprehensive Cancer Network recommends the consideration of ovarian transposition before pelvic radiation to prevent premature ovarian failure [10]. Ovarian transposition is a procedure used to move the ovaries out of radiation field and is usually performed during the primary surgery. Ovarian transposition may prevent premature ovarian failure but also has a negative effect such as abdominal pain, risk of injury to the ovarian vessels, development of ovarian cysts, bleeding, and torsion of vascular pedicle [11]. Therefore, ovarian transposition should only be performed in patients who are going to receive adjuvant therapy.
Ovarian transposition is a potential method for retaining fertility in young individuals who are receiving adjuvant therapy. However, this procedure does come with inherent hazards. Applying this treatment without considering the specific risks for each individual may result in avoidable surgical problems and potential damage to the blood vessels in the ovaries. The predictive model we have is crucial in this context as it provides a preoperative assessment of the patient’s likelihood of needing adjuvant therapy. This assessment is vital for making well-informed decisions regarding the surgical planning. By deviating from a generic strategy, we can precisely focus on ovarian transposition for those who are likely to benefit, therefore minimizing the possibility of unwanted intervention for others.
We aimed to establish a predictive model for adjuvant therapy risk in young women with early cervical cancer.
This study is an unplanned secondary analysis of a multicenter retrospective cohort study (Korean Gynecologic Oncology Group [KGOG] 1028), which was published previously [12]. KGOG 1028 included 1,441 patients with International Federation of Gynaecology and Obstetrics (FIGO) stage (2014) IB-IIA cervical cancer from nine institutions between January 2000 and December 2008. After the approval from the Institutional Review Boards of nine institutions were obtained, data were extracted from the KGOG 1028 database. The inclusion criteria were as follows: patients aged between 18 to 45 years; histology of squamous cell carcinoma or adenocarcinoma; and upfront radical or modified radical hysterectomy with pelvic and/or para-aortic lymph node sampling or dissection. The exclusion criteria were as follows: patients treated with neoadjuvant chemotherapy or primary radiation therapy; cervical cancer diagnosed incidentally after hysterectomy; and rare histology type such as neuroendocrine small cell carcinoma and clear cell carcinoma.
We collected preoperative variables such as age, body mass index, past disease history, preoperative laboratory findings, serum squamous cell cancer antigen (SCC-Ag) levels, FIGO stage, tumor size, and pathological variables including histological type, LVSI, DSI, LN involvement, PM involvement, and involvement of RM, and method approached for surgery. The classification of adjuvant therapy risk was deemed positive if any high-risk factor was detected, such as positive LN, positive RM, positive PM, or if the Sedlis criteria were met. Conversely, in the absence of these characteristics, the risk was defined as negative.
Association of preoperative variables with adjuvant therapy risk were examined using Student’s t-test or Mann-Whitney U-test for continuous variables and the Pearson’s chi-squared or Fisher’s exact test for categorical variables. Analyses were performed using SPSS statistical software version 25.0 (SPSS Inc., Chicago, IL, USA) and a value of P<0.05 was considered to be statistically significant.
After the missing values were imputed using the mean or median based on the distribution of each variable, a decision-tree model was constructed using the preoperative variables to predict the adjuvant therapy risk. Split was determined by the Gini index and the maximal depth and minimal samples leaf were optimized by Gridsearch using accuracy from five-fold cross-validation. Analyses were performed using Python ver3.7 in Google colaboratory website.
A total of 886 patients were included in this study. The clinicopathological characteristics of all patients are described in Table 1. The mean age of the population was 38.5±5.0 years, and most of the patients (73.7%) were stage IB1 with SCC (72.9%) histological type. Two-hundred-and-ninety (32.7%) patients had a tumor size <2 cm, 392 (44.2%) had a tumor sized 2-4 cm and 204 (23.0%) had a tumor size >4 cm. We found that 368 (41.5%) patients presented with outer one-third cervical stromal invasion, 231 (26.1%) with middle 1/3, and 287 (32.4%) with deep one-third thickness invasion. Positive involvement of the LVSI, LN, PM, and RM with cancer accounted for 337 (38.0%), 184 (20.8%), 69 (7.8%), and 26 (2.9%) patients, respectively. In addition, 792 (89.4%) and 94 (10.6%) patients underwent open and laparoscopic upfront surgeries, respectively.
Of 886 patients, 524 patients (59.1%) were adjuvant therapy risk (−) and 362 patients (40.9%) were adjuvant therapy risk (+). In the univariate analysis, hypertension history, white blood cell count and neutrophil count, stage, initial serum SCC-Ag level, tumor size, and method of approach for surgery were associated with adjuvant therapy risk (Table 2).
The final model divided a whole cohort into four subgroups according to tumor size and age (Fig. 1). Specifically, patients with a tumor size ≤2.45 cm had a 13.4% adjuvant therapy risk (49/367, calculated from the whole cohort). Those with a tumor size ≤3.85 cm and >2.45 cm had 43.3% risk (136/314). Those with a tumor size >3.85 cm and aged ≤39.5 years had 84.4% risk (92/109), and those with a tumor size >3.85 cm and aged >39.5 years had 88.5% risk (85/96). The classification accuracy from a five-fold cross-validation was 76.4%.
This study presents a novel decision-tree model for predicting the risk of requiring postoperative adjuvant therapy in young patients with early-stage cervical cancer. By stratifying patients based on tumor size and age, the model effectively personalizes surgical planning. Significantly, our results suggest a strong recommendation for ovarian transposition in patients with tumors >3.85 cm and age >39.5 years, given the 88.5% likelihood of requiring adjuvant radiation therapy.
Determining the necessity for ovarian transposition is challenging, particularly before the availability of pathological reports. Patients, especially younger women, should receive detailed counseling about the potential requirement of adjuvant radiotherapy following primary surgery. Currently, the decision to perform ovarian transposition is based on the estimated need for adjuvant therapy, which varies among oncologists due to the absence of standardized guidelines. Ovarian transposition aims to reduce pelvic radiation exposure and ovarian damage. A previous systematic review reported that ovarian function was successfully preserved in 61.7% of patients (ranging from 16.6% to 100%), after ovarian transposition followed by radiotherapy with or without chemotherapy [13]. While ovarian transposition is a valuable surgical method aimed at preserving ovarian function by minimizing radiation exposure, weighing this benefit against the risk of potential postoperative complications is critical. These complications may include abdominal pain, risk of injury to the ovarian vessels, development of ovarian cysts, bleeding, and torsion of vascular pedicle, which may affect ovarian function [11,13]. Therefore, offering ovarian transposition only if the patient is predicted to receive adjuvant treatment is desirable.
Our study has several limitations. First, the retrospective aspect of our study may have introduced potential bias. Second, as we relied only on the medical records, some missing data were observed for the variables, necessitating the use of imputed data. Additionally, the observed significance of variables like hypertension and white blood cell counts [14] could reflect the inherent variability of the multicenter design rather than that reflected by true clinical differences. As an unplanned secondary analysis, these variations may be random. Finally, this study does not reflect the FIGO stage 2018 since it used data from previously collected records. Importantly, the decision-tree model developed from this study warrants external validation to ascertain its effectiveness in other populations and healthcare settings.
Our research stands as the inaugural endeavor to develop a predictive model assessing the risk of requiring postoperative adjuvant therapy in early-stage cervical cancer, leveraging data collected from nine hospitals across Korea. This novel approach provides a tool that could substantially influence clinical decision-making, particularly regarding ovarian transposition in young patients. The widespread data collection underpins the model’s broader applicability and potential to guide clinicians in making informed, practical decisions that could enhance patient outcomes in gynecological oncology. In conclusion, the decision-tree model we have introduced serves as a pivotal aid in the presurgical assessment of young patients with early-stage cervical cancer, informing the critical decision on ovarian transposition. By integrating our model into clinical practice, gynecologists can adopt a more patient-centered approach, ensuring that interventions are tailored to the individual risks and benefits, thereby advancing the standard of care in gynecological oncology.
Table 1
Clinicopathological characteristics of all study patients
Table 2
Comparison of preoperative factors
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424.



2. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020;8:e191-203.


3. Meggiolaro A, Unim B, Semyonov L, Miccoli S, Maffongelli E, La Torre G. The role of pap test screening against cervical cancer: a systematic review and meta-analysis. Clin Ter 2016;167:124-39.

4. Yin L, Lu S, Zhu J, Zhang W, Ke G. Ovarian transposition before radiotherapy in cervical cancer patients: functional outcome and the adequate dose constraint. Radiat Oncol 2019;14:100.




5. Moon EK, Oh CM, Won YJ, Lee JK, Jung KW, Cho H, et al. Trends and age-period-cohort effects on the incidence and mortality rate of cervical cancer in Korea. Cancer Res Treat 2017;49:526-33.




6. Jewell EL, Kulasingam S, Myers ER, Alvarez Secord A, Havrilesky LJ. Primary surgery versus chemoradiation in the treatment of IB2 cervical carcinoma: a cost effectiveness analysis. Gynecol Oncol 2007;107:532-40.


7. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology group study. Gynecol Oncol 1999;73:177-83.


8. Rotman M, Sedlis A, Piedmonte MR, Bundy B, Lentz SS, Muderspach LI, et al. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys 2006;65:169-76.


9. Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys 2009;73:1304-12.



10. National Comprehensive Cancer Network. Cervical cancer (Version 1, 2021) [Internet]. In: Plymouth Meeting: National Comprehensive Cancer Network; c2020 [cited 2011 Nov 3]. Available from: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/ https://sukhin-oncology.com.ua/wp-content/uploads/2020/11/cervical2020.pdf
.
11. Moawad NS, Santamaria E, Rhoton-Vlasak A, Lightsey JL. Laparoscopic ovarian transposition before pelvic cancer treatment: ovarian function and fertility preservation. J Minim Invasive Gynecol 2017;24:28-35.


12. Paik ES, Lim MC, Kim MH, Kim YH, Song ES, Seong SJ, et al. Prognostic model for survival and recurrence in patients with early-stage cervical cancer: a Korean Gynecologic Oncology group study (KGOG 1028). Cancer Res Treat 2020;52:320-33.