 |
 |
- Search
| Obstet Gynecol Sci > Volume 67(1); 2024 > Article |
|
Abstract
Objective
Fetal uterine survival depends on maintaining an immune balance between the mother and fetus. This study aimed to investigate the correlation of blood peripheral natural killer (NK) cells and interferon-gamma (IFN-╬│) with recurrent recurrent implantation failure (RIF) and recurrent pregnancy loss (RPL).
Methods
In this case-control study, peripheral blood samples were obtained from three groups of RPL, RIF, and parous women without a history of abortion or infertility problems and analyzed by lymphocyte-based flow cytometry. Afterward, the levels of NK cells and IFN-╬│ were determined. All data were analyzed using one-way analysis of variance and nonparametric Kruskal-Wallis tests.
Results
The level of IFN-╬│ in the RPL group was significantly higher than that in parous women and the RIF group (P<0.05), whereas its level in the RIF group was not significantly different from the control group (P>0.05). A significant correlation was found between the levels of IFN-╬│ and NK cells in the RPL group (r=0.481; P=0.02). However, no significant correlation was found between the levels of IFN-╬│ and the active NK cells in the RPL group (P=0.08). Moreover, no significant correlation was found between the levels of NK cells (whether activated or not) and IFN-╬│ in the RIF patients (P>0.05).
As a growing medical and social problem, infertility affects 8.0-12.0% of reproductive-aged couples worldwide [1]. Recurrent pregnancy loss (RPL) is the failure of two or more clinical pregnancies before 20-24 weeks of gestation and affects approximately 2.5% of women [2]. Recurrent miscarriages may occur due to various factors, including uterine abnormalities, hormonal and metabolic disorders, antiphospholipid syndrome, cytogenetic abnormalities, and hereditary thrombophilia [3]. However, half of the abortions were idiopathic [3,4]. Footprints of the immune system are seen in most of these unexplained cases [5,6].
In vitro fertilization (IVF) is the most effective assisted reproductive technology (ART) for treating a wide variety of infertile cases [7]. However, approximately 30.0% of ART cases have resulted in live births [8]. Recurrent failure of implantation (RIF) may be caused by reduced endometrial acceptance, uterine polyps, myomas, congenital anomalies, endometriosis, thrombophilia, or male or fetal problems. Increasing evidence suggests that unexplained RIF and RPL cases may be mediated by insufficient and excessive endometrial inflammatory responses during the window of implantation [6,8-10].
The survival of an allogeneic fetus in the uterus depends on maintaining an immune balance between the mother and fetus. Achieving and maintaining this safety balance has been the subject of considerable research [11,12]. Natural killer cells (NK cells) are the main immune cells involved in maintaining a normal pregnancy and have been identified as safety factors associated with reproductive success [13]. The NK cell count fluctuates during different menstrual cycle stages (likely increases after ovulation, especially during the secretory phase) [14]. NK cells comprise 5.0-10.0% peripheral blood lymphocytes and 70.0-90.0% uterine lymphocytes. NK cells express CD56 and CD16 markers; unlike T cells, they do not express CD3 [15]. In early pregnancy, these cells play a key role in implantation, particularly in vascular regeneration and trophoblast attacks. Also, NK cells play a role through inflammatory cytokines such as granulocyte-macrophage colony-stimulating factor, leukemia inhibitory factor, interleukin (IL)-1, IL-6, and IL-11 [16-18]. Therefore, NK cells facilitate healthy development during pregnancy by maintaining a balance between placental function and fetal needs. Some cytokines positively promote pregnancy, while others are toxic to the fetus, including IL-12 and interferongamma (IFN-╬│) [19].
IFN-╬│ is a homodimeric protein produced by innate immune cells such as NK cells and CD4+ and CD8+ T lymphocytes. NK cells are the main source of IFN-╬│ production during the early stages of the immune response, stimulating IL-12 production from macrophages. These cytokines then increase IFN-╬│ production. Therefore, large amounts of IFN-╬│ are produced even before the onset of adaptive immunity [20,21]. The prognostic value of peripheral NK cells as a screening test for women with infertility is still controversial [22].
This study selected three surface markers of NK cells in the peripheral blood of women, CD56, CD16, and CD69, were selected for examination. Moreover, the amount of peripheral IFN-╬│ was measured, and the correlation between IFN-╬│ levels and NK cells was assessed to determine the role of peripheral blood NK cells in recurrent miscarriage and IVF failure.
In this case-control study, all samples were obtained from women referred to the infertility ward of Imam Khomeini Hospital of Ahvaz Jundishapur University for infertility from March 1, 2021 to January 1, 2022. This study was approved by the Ethics Committee of the Research Department of Ahvaz Jundishapur University of Medical Sciences (ethical code: IR.AJUMS.REC.2020.096). Written informed consent was obtained from all patients before the study initiation. All procedures in this clinical study involving human participants were in accordance with the ethical standards of the National Research Committee and the 2008 Declaration of Helsinki and its later amendments.
A total of 57 peripheral blood samples were collected from women aged 18-40 years divided into three groups (22 women with RPL, 18 with RIF, and 17 parous women). Blood samples were collected during the midluteal phase, and blood tests were conducted during the same luteal phase of the menstrual cycle. The control group included parous women with no history of abortion or infertility. Women with a history of autoimmune diseases such as antiphospholipid antibody syndrome, infectious diseases, diabetes, chromosomal abnormalities, and immunodeficiency were excluded from the study. In addition, patients with anatomical, hormonal, or abnormal chromosomal abortions, those positive for toxoplasma, rubella, cytomegalovirus, and herpes simplex, or both, were excluded from the study.
RPL was defined as three consecutive pregnancy losses before 20 weeks from the last pregnancy, in which all the following outcomes were normal: thyroid function, pelvic ultrasonography and hysterosalpingogram, parental karyotypes, anti-cardiolipin and antiphospholipid antibodies, fetal bovine serum, lupus anticoagulant, prolactin, progesterone, estrogen, testosterone, follicle-stimulating hormone, free androgen index, and prothrombotic risk factors (factor V Leiden, activated protein-C resistance, and prothrombin mutations). They had a history of at least two sequential spontaneous miscarriages without the involvement of any other infertility factors [23].
RIF is defined as the failure of post-IVF implantation after two stages of high-quality egg transfer. Women with RIF-related infertility had an infertility history of more than 1 year with normal thyroid function, serum prolactin, and hysterosalpingography, while their male partners had a normal sperm count, motility, and morphology according to the World Health Organization standards.
After obtaining informed consent, 5 mL of heparinized peripheral blood was drawn from each person and divided into two parts: 2 mL was transferred to a heparin tube for flow cytometry and 3 mL to a clot tube for serum preparation and enzyme-linked immunosorbent assay (ELISA) for IFN-╬│ measurement.
Specific antigenic indices of lymphocytes on the immune system cells can be distinguished by flow cytometry using monoclonal antibodies against these indices [22]. Monoclonal antibodies against markers CD16 and CD56 detecting NK cells and CD69 identifying active NK cells were obtained from BioLegend (Bio-Techne, Minneapolis, MN, USA). The IFN-╬│ ELISA kit and Lysis Buffer were purchased from Bioscience (Bio-Techne) and DACO (DAKO Company, Santa Clara, CA, USA), respectively. Serum levels of IFN-╬│ were measured by using a Bioscience ELISA kit.
Immediately after the lysis of red blood cells using a buffer lysis solution, blood leukocytes were stained with fluorescent antibodies conjugated with specific fluorescent materials against CD16, CD56, CD69, and CD69 markers. These were analyzed using flow cytometry in the lymphocyte basin, and the percentages of activated NK and NK cells were determined. Flow cytometry data were analyzed using FlowJo software version 10 (Bio-Techne), and graphs were plotted using GraphPad Prism 8 (Bio-Techne, Minneapolis, MN, USA) (Fig. 1). Finally, the data were averaged over the cells CD56+ for lymphocytes; CD16+ and CD56+ for NK cells; CD16+, CD56+, and CD69+ for active NK cells; and CD16-, CD56+, and CD69- for non-NK cell lymphocytes using SPSS software version 26 (DAKO Company).
The sample size was estimated using an ╬▒ value of 0.05 and a study power of 89.8% based on the statistical methods in medical research by Armitage et al. [24]. One-way analysis of variance, nonparametric Kruskal-Wallis tests, and SPSS software version 26 (DAKO Company) were used for data analysis. P<0.05 is considered as statistically significant.
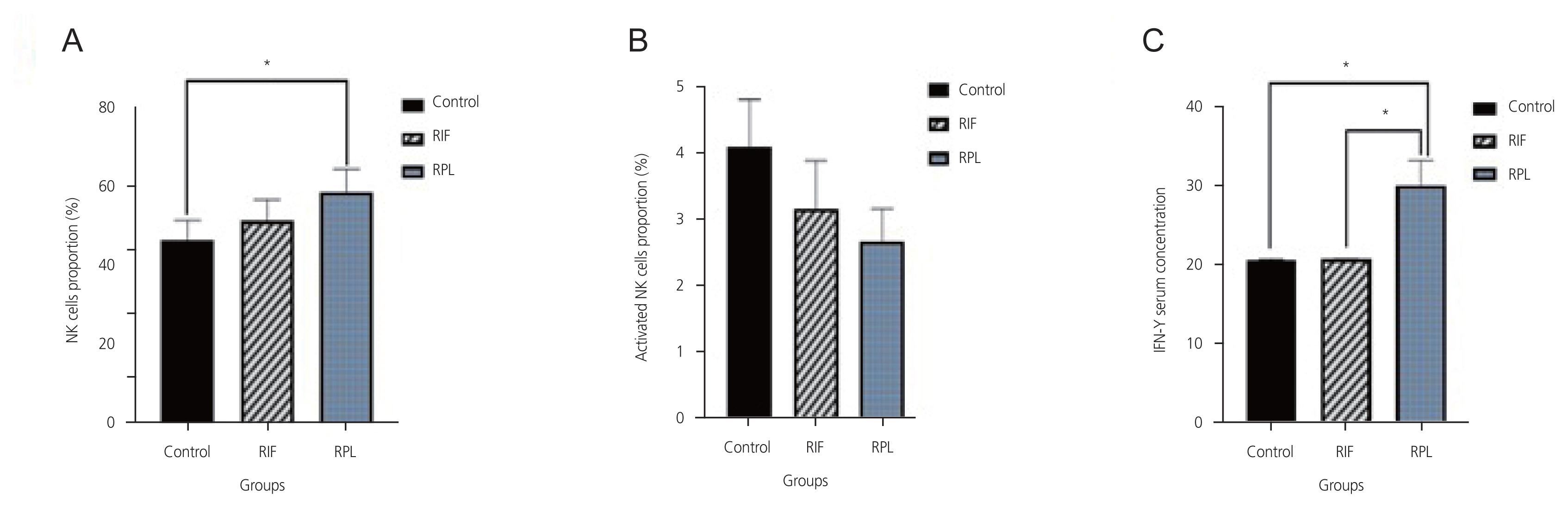
The mean numbers of NK cells (CD16+ and CD56+) in the RPL group were higher than those in the control group (P=0.02). However, the mean numbers of active NK cells in the RIF and RPL groups were not significantly different from those in the control group (P=0.3; Table 1). Although the mean number of NK cells in RIF patients was higher than that in the control group, the difference was insignificant (P>0.05; Table 1).
The level of IFN-╬│ in the RPL group was significantly higher than in the control and RIF groups (P<0.05). In contrast, its level in the RIF group was insignificantly different from the control group (P>0.05; Table 1).
We investigated the correlation between the level of IFN-╬│ and the level of NK cells to determine the exact role of immune response in IVF and pregnancy outcomes. We also explored the diagnostic sensitivity of IFN-╬│. In the RPL group, there was a significant correlation between the levels of IFN-╬│ and NK cells (r=0.481; P=0.02). However, there was no significant correlation between the levels of IFN-╬│ and the active NK cells in the RPL group (P=0.08; Table 2). No significant correlation was found between the levels of NK cells (whether activated or not) and IFN-╬│ in the RIF patients (P>0.05; Table 2 and Fig. 2).
Our findings showed no difference in NK cells and IFN-╬│ levels between RIF patients and controls. Moreover, no correlation was found between these two markers in RIF patients, implying that immunosuppression and autoimmunity do not play a role in implantation failure during IVF. However, the significantly higher levels of NK cells and IFN-╬│ in the RPL group than those in the control group and RIF patients imply that autoimmunity and IFN-╬│-related cytotoxicity may be involved in clinical pregnancy loss before 20-24 weeks of gestation.
NK cells play an important role in female reproduction, and their effects on RPL and RIF have been demonstrated in previous studies. In a case-control study conducted by Zhang et al. [25], NK cells CD3- and CD56+ were examined for expression of granzyme B, granulation, and perforin, and NKG2D, NKP30, NKP46, CD158a, and CD158b receptors in 27 patients with RIF. The cytotoxicity of peripheral blood NK cells was investigated by measuring the expression of granzyme B, granulation, perforin, and the NKG2D, NKP30, NKP46, CD158a, and CD158b receptors. The levels of these factors in RIF patients were not significantly different from those in the control group. RIF was not associated with significant changes in the number and functions of NK cells [25]. Our findings confirmed no significant correlation between RIF and the levels of peripheral blood or active NK cells.
Thum et al. [26] evaluated 138 patients with infertility treated with IVF. Their results showed no significant differences between the numbers of peripheral blood types of NK, B, and T cells and no correlation between them and IVF or pregnancy outcomes [26]. Our findings are consistent with those reported by Zhang et al. [25] and Thum et al. [26]. These studies indicated that peripheral blood NK cells are not associated with RIF. In contrast, studies by Tuckerman et al. [27] and Mardanian et al. [28] showed an association between endometrial NK cells and infertility in patients with RIF. Tuckerman et al. [27] proposed that the increase in CD56+ cell density in the endometrium of women with RIF indicates a direct role of CD56+ cells in the implantation process. However, we only examined peripheral blood NK cells, not endometrial ones. Mardanian et al. [28] indicated a significant increase in CD56+ and CD16+ cell levels in women with IVF failure compared with those with successful IVF. In our study, the total number of CD56+ and CD16+ cells was not associated with recurrent IVF failure. Such contradictory results may be due to genetic and phenotypic differences in populations from different regions of the world or differences in measurement methods.
We examined the CD69 marker to identify active NK cells, while DonsŌĆÖkoi et al. [29] evaluated the expression of NKp46 in peripheral blood NK cells in women with a history of RIF. They reported that the percentage of NKp46+ NK cells in women with RIF was significantly higher than in the control group [29].
In a study by Kuon et al. [30], no correlation was found between the number of peripheral blood NK cells and uterine NK, while an increase was detected in peripheral lymphocytes CD45+, CD3-, DR+, CD45+, CD3+, CD8+, DR+, and uNK. This suggests that NK, B, and T-activated cells provide cytokines for uNK cell differentiation [30]. Our findings confirm these results [30], meaning that the peripheral blood NK cells are involved in recurrent miscarriage by increasing IFN-╬│ levels.
Another reason for the mismatch between the increased levels of IFN-╬│ and activated NK cells could be the cytokine synthesis by cells other than activated NK cells. For example, Th1 cells can synthesize IFN-╬│, and an increase in Th1 activity followed by an increase in IFN-╬│ production leads to recurrent miscarriage [31,32]. Other cells producing IFN-╬│ are NKT cells, whose role in recurrent miscarriage has been shown in previous studies [33,34].
In this study, the CD69 receptor was assessed to identify activated NK cells. The difference between our results and those of other studies regarding the lack of an increase in activated NK cells may be due to the examination of different surface markers in NK cells in different studies [22,35,36].
Overall, it is important to understand that NK cell receptors do not accurately represent the specific immune responses to pregnancy, and the count and activity of NK cells can change based on age, menstruation, hormones, stress, and physical activity on the day of sampling [37-39].
This study is one of the rare studies that compared both markers of NK cells and IFN-╬│ between three groups of women with RIF, RPL, and parous women, not merely two groups. Moreover, the fluorescence minus one (FMO) method used in the flow cytometry test in this study has several advantages over the isotype control method used in most previous studies. Isotype controls cannot provide gating controls, while FMO controls can identify gating boundaries. Isotype controls are specifically appropriate for determining the background due to nonspecific antibody binding and cannot distinguish positive from negative cells [40].
This study has several strengths. First, it evaluated both markers of NK cells and IFN-╬│ between three groups of women with RIF, RPL, and parous women. Second, the FMO controls were used in this study. However, the single-center nature of the study and the small sample size of each group limit the generalizability of the results. In this study, the patients with known conditions affecting NK cells, abortion, and infertility were excluded. Nevertheless, these conditions should be standardized as much as possible in future research. Laboratory results may be highly affected by the type of technique used, the genetic characteristics of the population, type of samples, and sampling method. The neurological and hormonal statuses and their effects on NK cells may be useful in these patients to achieve a more comprehensive understanding of the function of these cells. Therefore, future research should study patients with RIF and RPL before pregnancy, at a certain period of the menstrual cycle, and during pregnancy.
Our findings proposed that RIF is not associated with the levels of the peripheral blood NK Cells and IFN-╬│, implying that immune dysfunction may not be involved in implantation failure during IVF. Peripheral blood NK cells may be involved in recurrent miscarriage by increasing IFN-╬│ levels and inducing cytotoxicity. This requires a more precise investigation in future large-scale studies. Our findings on the possible involvement of immune dysfunction and IFN-╬│-related cytotoxicity in inducing pregnancy loss provide a prospect for diagnostic, preventive, and therapeutic research on infertility.
Given the possible role of peripheral blood NK cells and IFN-╬│ levels in inducing cytotoxicity and the recurrent miscarriage pointed out in this study, measurement of the levels of these markers in pregnant women can be clinically useful for monitoring immune balance and preventing adverse outcomes.
Notes
Ethical approval
Participation in this study was voluntary, and all participants signed an informed consent form. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board and Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (ethical code: IR.AJUMS.REC.2020.096).
Fig.┬Ā1
(A) Lymphocyte gates in the total cell population; (B) Isolation of NK cells (CD16+ and CD56+) from the rest of the lymphocyte population using conjugated monoclonal antibody with fluorescent materials specific for CD56 and CD16; (C) FMO control gating for CD16 to detect CD16+ cells area; (D) FMO control gating for CD69 to detect CD69+ cells area; (E) a test sample showing cell populations. Area Q1: CD69+ and CD56+; Area Q2: CD69+, CD16+, and CD56+; Area Q3: CD56+ and CD16+; Area Q4: CD56+, CD16-, and CD69-. SSC-H, side scatter height; FSC-H, forward scatter height; NK cells, natural killer cells; FMO, fluorescence minus one.
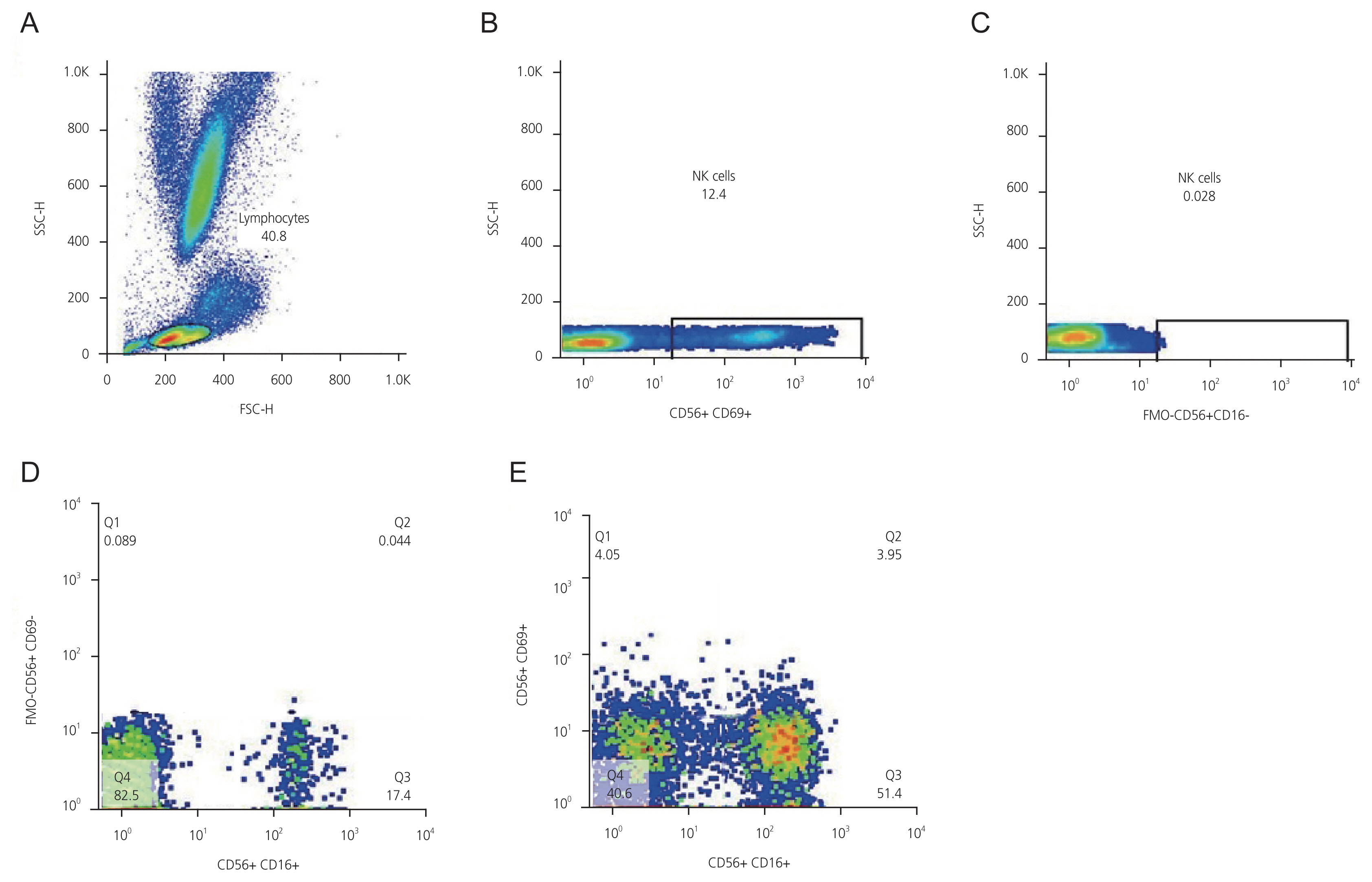
Fig.┬Ā2
Comparison of different levels of peripheral blood NK cells (A), activated NK cells (B), and IFN-╬│ (C) in various studied groups. *P-value <0.05 is significant. NK cells, natural killer cells; RIF, recurrent failure of implantation; RPL, pregnancy loss; IFN-╬│,
Table┬Ā1
Comparison of mean percentages of peripheral blood NK cells, active NK cells, and IFN-╬│ level (pg/mL) in three groups
| RIF group | RPL group | Control group | P-value | |
|---|---|---|---|---|
| NK cells (CD16+ and CD56+) | 8.45┬▒1.157 | 12.00┬▒1.221 | 7.467┬▒0.84 | 0.02a) |
| Active NK cells (cD16+, CD56+, and CD69+) | 3.204┬▒0.721 | 4.141┬▒0.713 | 2.70┬▒0.493 | 0.3 |
| IFN-╬│ | 20.88┬▒0.094 | 30.22┬▒3.161 | 20.73┬▒0.706 | 0.01a) |
Table┬Ā2
Correlation between the levels of IFN-╬│, peripheral blood NK cells, and active NK cells in RPL and RIF groups
| RPL group | RIF group | |||
|---|---|---|---|---|
|
|
|
|||
| Correlation coefficient | P-value | Correlation coefficient | P-value | |
| Correlation between IFN-╬│ level and NK cells level | 0.481 | 0.027a) | ŌłÆ0.188 | 0.453 |
|
|
||||
| Correlation between IFN-╬│ level and active NK cells level | 0.718 | 0.083 | ŌłÆ0.386 | 0.112 |
References
1. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem 2018;62:2-10.


2. Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ. Recurrent pregnancy loss. Nat Rev Dis Primers 2020;6:98.



3. El Hachem H, Crepaux V, May-Panloup P, Descamps P, Legendre G, Bouet PE. Recurrent pregnancy loss: current perspectives. Int J Womens Health 2017;9:331-45.


4. Scantamburlo VM, Linsingen RV, Centa LJR, Toso KFD, Scaraboto D, Araujo E J├║nior, et al. Association between decreased ovarian reserve and poor oocyte quality. Obstet Gynecol Sci 2021;64:532-93.




5. Kim SG, Paek MY, Ko IG. Peripheral blood level of natural killer cells in pregnant women with recurrent spontaneous abortion during the 6-12 weeks gestation. Arch Med Health Sci 2019;7:191-4.

6. Zhu LY, Chen X, Xu ZZ, Xu L, Mao T, Zhang H. Changes and clinical significance of peripheral blood helper T lymphocyte and natural killer (NK) cells in unexplained recurrent spontaneous abortion (URSA) patients after abortion and successful pregnancy. Clin Exp Obstet Gynecol 2015;42:62-6.


7. Zargar M, Pazhouhanfar R. Effects of intrauterine autologous platelet-rich plasma infusions on outcomes in women with repetitive in vitro fertilization failures: a prospective randomized study. Clin Exp Obstet Gynecol 2021;48:179-84.

8. Genest G, Banjar S, Almasri W, Beauchamp C, Benoit J, Buckett W, et al. Immunomodulation for unexplained recurrent implantation failure: where are we now? Reproduction 2023;165:R39-60.


9. Yuan J, Li J, Huang SY, Sun X. Characterization of the subsets of human NKT-like cells and the expression of Th1/Th2 cytokines in patients with unexplained recurrent spontaneous abortion. J Reprod Immunol 2015;110:81-8.


10. Park S, You YA, Yun H, Choi SJ, Hwang HS, Choi SK, et al. Cervicovaginal fluid cytokines as predictive markers of preterm birth in symptomatic women. Obstet Gynecol Sci 2020;63:455-63.




11. Iske J, Elkhal A, Tullius SG. The fetal-maternal immune interface in uterus transplantation. Trends Immunol 2020;41:213-24.



12. Fox C, Morin S, Jeong JW, Scott RT Jr, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertil Steril 2016;105:873-84.



13. Sacks G, Finkelstein E. Natural killer cells and reproductive success. Am J Reprod Immunol 2021;85:e13291.



14. Mahajan D, Sharma NR, Kancharla S, Kolli P, Tripathy A, Sharma AK, et al. Role of natural killer cells during pregnancy and related complications. Biomolecules 2022;12:68.



15. Amand M, Iserentant G, Poli A, Sleiman M, Fievez V, Sanchez IP, et al. Human CD56dimCD16dim cells as an individualized natural killer cell subset. Front Immunol 2017;8:699.



16. Sharma S. Natural killer cells and regulatory T cells in early pregnancy loss. Int J Dev Biol 2014;58:219-29.



17. Amirian A, Mahani MB, Abdi F. Role of interleukin-6 (IL-6) in predicting gestational diabetes mellitus. Obstet Gynecol Sci 2020;63:407-16.




18. Gupta S, Gaikwad HS, Nath B, Batra A. Can vitamin C and interleukin 6 levels predict preterm premature rupture of membranes: evaluating possibilities in North Indian population. Obstet Gynecol Sci 2020;63:432-9.




19. Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 2018;49:397-412.



20. Choghakabodi PM, Pouladzadeh M, Haybar H, Keikhaei B, Hossein Nezhad K, Jalali Far MA, et al. Biological whistleblowers for silent myocardial ischemia: diagnostic and prognostic approach. Recenti Prog Med 2020;111:415-25.

21. Mahy Brian WJ, vanRegenmortel Marc HV. Desk encyclopedia of general virology. In: Griffin DE, editors. Cytokines and chemokines. 3rd ed. Atlanta: Academic Press; 2008. p. 620-4.
22. Ghafourian M, Karami N, Khodadadi A, Nikbakht R. Increase of CD69, CD161 and CD94 on NK cells in women with recurrent spontaneous abortion and in vitro fertilization failure. Iran J Immunol 2014;11:84-96.

23. Bashiri A, Halper KI, Orvieto R. Recurrent implantation failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol 2018;16:121.




24. Armitage P, Berry G, Matthews JNS. Statistical methods in medical research. 4th ed. London: John Wiley & Sons; 2002.
25. Zhang H, Huang C, Chen X, Li L, Liu S, Li Y, et al. The number and cytotoxicity and the expression of cytotoxicity-related molecules in peripheral natural killer (NK) cells do not predict the repeated implantation failure (RIF) for the in vitro fertilization patients. Genes Dis 2019;7:283-9.



26. Thum MY, Bhaskaran S, Bansal AS, Shehata H, Ford B, Sumar N, et al. Simple enumerations of peripheral blood natural killer (CD56+ NK) cells, B cells and T cells have no predictive value in IVF treatment outcome. Hum Reprod 2005;20:1272-6.


27. Tuckerman E, Mariee N, Prakash A, Li TC, Laird S. Uterine natural killer cells in peri-implantation endometrium from women with repeated implantation failure after IVF. J Reprod Immunol 2010;87:60-6.


28. Mardanian F, Kazeroonizadeh M, Rashidi B. Evaluation of CD56(dim) and CD56(bright) natural killer cells in peripheral blood of women with IVF failures. Iran J Reprod Med 2015;13:577-82.


29. DonsŌĆÖkoi BV, Osypchuk DV, Chernyshov VP, Khazhylenko KG. Expression of natural cytotoxicity receptor NKp46 on peripheral blood natural killer cells in women with a history of recurrent implantation failures. J Obstet Gynaecol Res 2021;47:1009-15.

30. Kuon RJ, Vomstein K, Weber M, M├╝ller F, Seitz C, Wallwiener S, et al. The ŌĆ£killer cell storyŌĆØ in recurrent miscarriage: association between activated peripheral lymphocytes and uterine natural killer cells. J Reprod Immunol 2017;119:9-14.


31. Kwak-Kim JYH, Gilman-Sachs A, Kim CE. T helper 1 and 2 immune responses in relationship to pregnancy, nonpregnancy, recurrent spontaneous abortions and infertility of repeated implantation failures. Chem Immunol Allergy 2005;88:64-79.


32. Piccinni MP. T cells in normal pregnancy and recurrent pregnancy loss. Reprod Biomed Online 2007;14:Spec 1. 95-9.


33. Shimada S, Kato EH, Morikawa M, Iwabuchi K, Nishida R, Kishi R, et al. No difference in natural killer or natural killer T-cell population, but aberrant T-helper cell population in the endometrium of women with repeated miscarriage. Hum Reprod 2004;19:1018-24.


34. Schoenborn JR, Wilson CB. Regulation of interferongamma during innate and adaptive immune responses. Adv Immunol 2007;96:41-101.

35. Mikhailova VA, Khokhlova EV, Bazhenov DO, Agnaeva AO, Kozyreva AR, Bespalova ON, et al. Changes in expression of Ki-67, CD16 and CD56 by natural killer cells from peripheral blood mononuclear cells in the setting of recurrent miscarriage after in vitro culturing in the presence of trophoblast cells and IL-2. Cytotechnology 2019;71:861-71.




36. Yougbar├® I, Tai WS, Zdravic D, Oswald BE, Lang S, Zhu G, et al. Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia. Nat Commun 2017;8:224.




37. Zhu L, Aly M, Kuon RJ, Toth B, Wang H, Karakizlis H, et al. Patients with idiopathic recurrent miscarriage have abnormally high TGF├¤+ blood NK, NKT and T cells in the presence of abnormally low TGF├¤ plasma levels. BMC Immunol 2019;20:10.




38. Fiuza-Luces C, Padilla JR, Valent├Łn J, Santana-Sosa E, Santos-Lozano A, Sanchis-Gomar F, et al. Effects of exercise on the immune function of pediatric patients with solid tumors: insights from the PAPEC randomized trial. Am J Phys Med Rehabil 2017;96:831-7.






















