Survival probabilities and prognostic factors of pregnancy-associated breast cancer in Malaysian women
Article information
Abstract
Objective
Pregnancy-associated breast cancer (PABC) is a rare cancer. This study aimed to determine the survival probabilities and prognostic factors in patients with PABC.
Methods
A retrospective cohort study was conducted in two tertiary care hospitals in Kota Bharu. We included all patients with breast cancer who were diagnosed by histopathology while pregnant or within 2 years post-partum from 2001 through 2020. We matched patients with PABC to non-pregnant patients with breast cancer by age and year of diagnosis. The data were analyzed using Cox proportional hazard regression.
Results
A total of 35 cases of PABC and 70 non-PABC controls were recruited. The 3-year, 5-year, and 10-year survival probabilities for patients with PABC were 58.6%, 47.54%, and 38.03%, respectively. The patients with PABC had a non-significant difference in survival probabilities compared with non-PABC patients. The significant prognostic factors of PABC were age (adjusted hazard ratio [aHR], 0.91; 95% confidence interval [CI], 0.86–0.96; P=0.001), advanced stage of cancer (aHR, 9.97; 95% CI, 3.96–25.2; P<0.001), and no surgery (aHR, 3.16; 95% CI, 1.01–9.85; P=0.047). Pregnancy was not found to be an independent factor in the prognosis of PABC (aHR, 0.72; 95% CI, 0.39–1.28; P=0.266).
Conclusion
Women diagnosed with PABC had similar survival probabilities compared with non-PABC patients. Pregnancy was not an independent prognostic factor for breast cancer. This information can be useful when women with breast cancer are counseled and supported with the option of beginning treatment with pregnancy continuation.
Introduction
Cases of breast cancer in Malaysia are increasing at an alarming rate. One in every 19 Malaysian women is at risk of developing breast cancer [1]. Nearly half of all patients with breast cancer are under the age of 50 years [1]. The 50–59 years age range has the highest age-specific incidence of breast cancer in Malaysia [2].
Pregnancy-associated breast cancer (PABC) is described as breast cancer identified during the gestational or postpartum periods [3]. It is a rare condition that has received limited attention from researchers in low- and middle-income countries. Breast cancer affects approximately 4.0% of women under 45 years of age during pregnancy or the first year after giving birth [4].
As summarized in a recent review [5], potential mechanisms that might be associated with the pathophysiology of PABC are hormonal changes during pregnancy (increased levels of estrogen and progesterone), enhanced inflammatory responses, pregnancy-associated immune tolerance, high oncogene expression, and breast involution, which can prompt and enhance oncogenic developments.
The association of pregnancy with breast cancer prognosis is widely debated in the literature. According to some studies, pregnancy may lead to a poor prognosis, and women with PABC have a poorer survival outcome than their non-pregnant counterparts [6–10]. However, a few studies have found no significant difference in the survival rates of pregnant and non-pregnant breast cancer cohorts [11–14].
The clinical features of PABC tend to be aggressive, presenting with large tumors, hormone-negative tumors, and advanced cancer stage [3,4,13,15]. While the difference in clinical characteristics between the groups is considered to lead to low survival in women with PABC, a few studies [16] suggest that a delay in diagnosis may also be a key factor in prognosis. Symptoms of breast cancer during pregnancy may be ignored, attributed to another pregnancy-related condition, or otherwise delayed in identification due to unsuspected cancer [17]. This can further cause a delay in both the diagnosis and treatment of breast cancer.
In a retrospective French study [14], patients with histologically proven, invasive breast cancer that was detected during pregnancy were recruited and matched with controls according to age, clinical T stage, hormone receptor, and neoadjuvant chemotherapy. Relapse was documented in 48.2% of pregnant patients with breast cancer and 37.5% of non-pregnant patients. There was no significant difference in survival between the patients with PABC and the controls. The 5-year overall survival (OS) rates of the two groups were 83.1% and 85.5%, respectively.
Similarly, in a study by Zhang et al. [18], 41 PABC cases were matched (1:1) with non-PABC controls according to similarities in breast cancer stage, age, and year of diagnosis. The pregnant group had considerably more progesterone receptors (PR)-and triple-negative tumors compared with those of the non-PABC group. The median OS in the PABC group was 82.8 months vs. 80.1 months in the non-PABC group.
Research on PABC in Asian countries is scarce. Therefore, we conducted this study to determine the survival probabilities and prognostic factors of patients with PABC in Kelantan, Malaysia. This study may help create awareness among clinicians and patients regarding the possible risks that are posed by pregnancy in breast cancer and subsequently encourage better and more frequent healthcare services.
Materials and Methods
This was a retrospective cohort study. The PABC cohort consisted of women who were diagnosed with PABC and registered in the database from January 2001 to December 2020. A total of 35 patients with PABC files and breast cancer records were reviewed. PABC was defined as breast cancer that was diagnosed during the period of pregnancy or within 24 months of delivery [3]. Post-partum cases were confirmed thorough review of the medical files. Breast cancer diagnoses in women who delivered in the previous 1–2 years were included as post-partum cases.
The inclusion criteria for the PABC cohort were confirmed diagnosis of breast cancer by histopathological examination and a history of pregnancy. The inclusion criteria for the non-PABC cohort were confirmed diagnosis of breast cancer by histopathological examination and no recent history (within 24 months) of gestation or parturition while having signs and symptoms of breast cancer, during and after diagnosis [3]. The exclusion criteria for both groups were diagnosis of any other type of cancer and incomplete medical record of 30.0% variables.
The population for PABC was small; therefore, all PABC cases were analyzed. While non-PABCs were selected by matching patient age and the year of diagnosis, we matched 35 PABC cases with 70 non-PABC cases by age (±1 years) and year of diagnosis (±3 years). Cohorts of women with breast cancer, one pregnant cohort and another non-pregnant cohort, were evaluated for their survival outcomes. Socio-demographic and clinical data from the patients’ medical files were reviewed and recorded as per requirement. The time from the date of diagnosis to the event (died)/non-event (alive or lost to follow-up) or to the end of follow-up was measured. Mortality status was coded as 1, and censor was coded as 0, with the event being death or mortality and censor being survival, loss to follow-up, or the end of the study. The survival status was checked with the National Registration Department by matching the identification card numbers of the samples. A proforma was created to collect data on socio-demographic characteristics, clinical features, treatments, survival, and pregnancy characteristics.
SPSS version 26 (IBM Corp., Armonk, NY, USA) and STATA version 14 (Stata Statistical Software, College Station, TX, USA) were used for the statistical analyses. Descriptive statistics were performed on the patient characteristics, clinical data, and treatment and pregnancy information. All numerical variables were assessed for their normality distributions. The mean and standard deviation of the numerical variables with normal distributions were reported. The medians and interquartile ranges of numerical variables with skewed/non-normal distributions were reported. The frequencies of observation and their percentages were reported for the categorical variables. The means and medians were compared using the independent t-test and the Wilcoxon Mann-Whitney test, respectively. Proportions were compared using the chi-square or Fisher’s exact tests.
The Kaplan-Meier method was applied to calculate the OS probabilities. The Log-Rank test was performed to compare the differences in survival probabilities. The Cox regression model was utilized to determine the hazard ratios (HRs) of the independent variables. Simple Cox regression analysis was conducted for all independent variables to identify the variable related to prognosis. The independent variables with a P-value of less than 0.25 were included in the multiple Cox model. Cox proportional hazard regression was performed to determine the prognosis effect of pregnancy. The crude HRs, adjusted hazard ratios (aHRs), 95% confidence intervals (CIs), Wald statistics, and the corresponding P-values of selected variables from the final model of multiple Cox regression analysis were reported [19].
The principles of the Helsinki Declaration and the Malaysian good clinical practice guidelines were followed by all researchers who were involved in this study.
Results
1. Comparison of socio-demographic, clinical, and treatment characteristics between patients with PABC and non-PABC patients
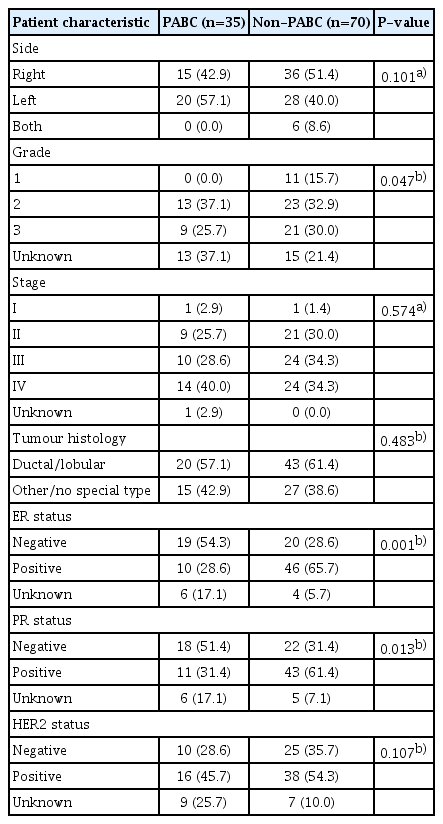
A total of 35 patients in the PABC group and 70 patients in the non-PABC group were identified during the study period. In the PABC group, 27 patients with breast cancer were diagnosed during pregnancy and eight were diagnosed within 24 months of giving birth. Table 1 shows the median follow-up as 30.93 months in the PABC group and 36.57 months in the non-PABC group. The mean age at diagnosis of women with PABC was 34 years (standard deviation [SD], 4.77) and that of the non-PABC group was 35.81 years (SD, 5.10). There were no significant differences between the PABC and non-PABC groups in marital and employment status.
Table 2 shows the comparison of clinical characteristics. Ductal/lobular carcinoma was common in both the PABC and non-PABC groups. estrogen receptor (ER)-negative tumors were more prevalent in patients with PABC (54.3% vs. 28.6%; P=0.001). Similarly, patients with PABC had more PR-negative tumors (51.4%) than those without PABC (31.4%); this difference was statistically significant (P=0.013).
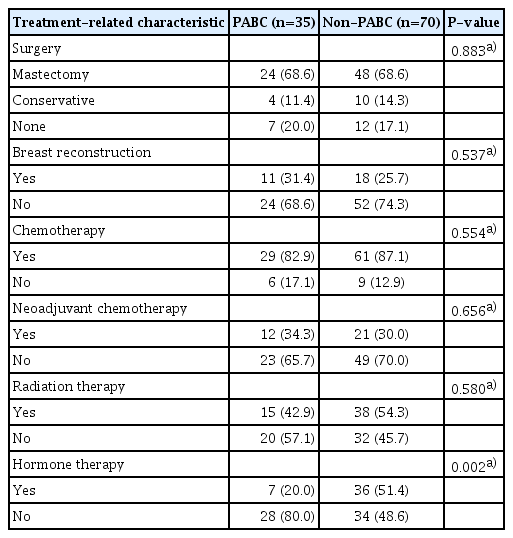
Table 3 depicts mastectomy as the chosen surgery method in both groups. Radiation therapy was administered frequently in the non-PABC cohort. There was no significant difference in treatment mode between the two groups except for hormone therapy. In the non-PABC group, 51.4% of patients received hormone therapy compared with 20.0% of patients in the PABC group (P=0.002).
2. Comparison of survival between the PABC and non-PABC groups
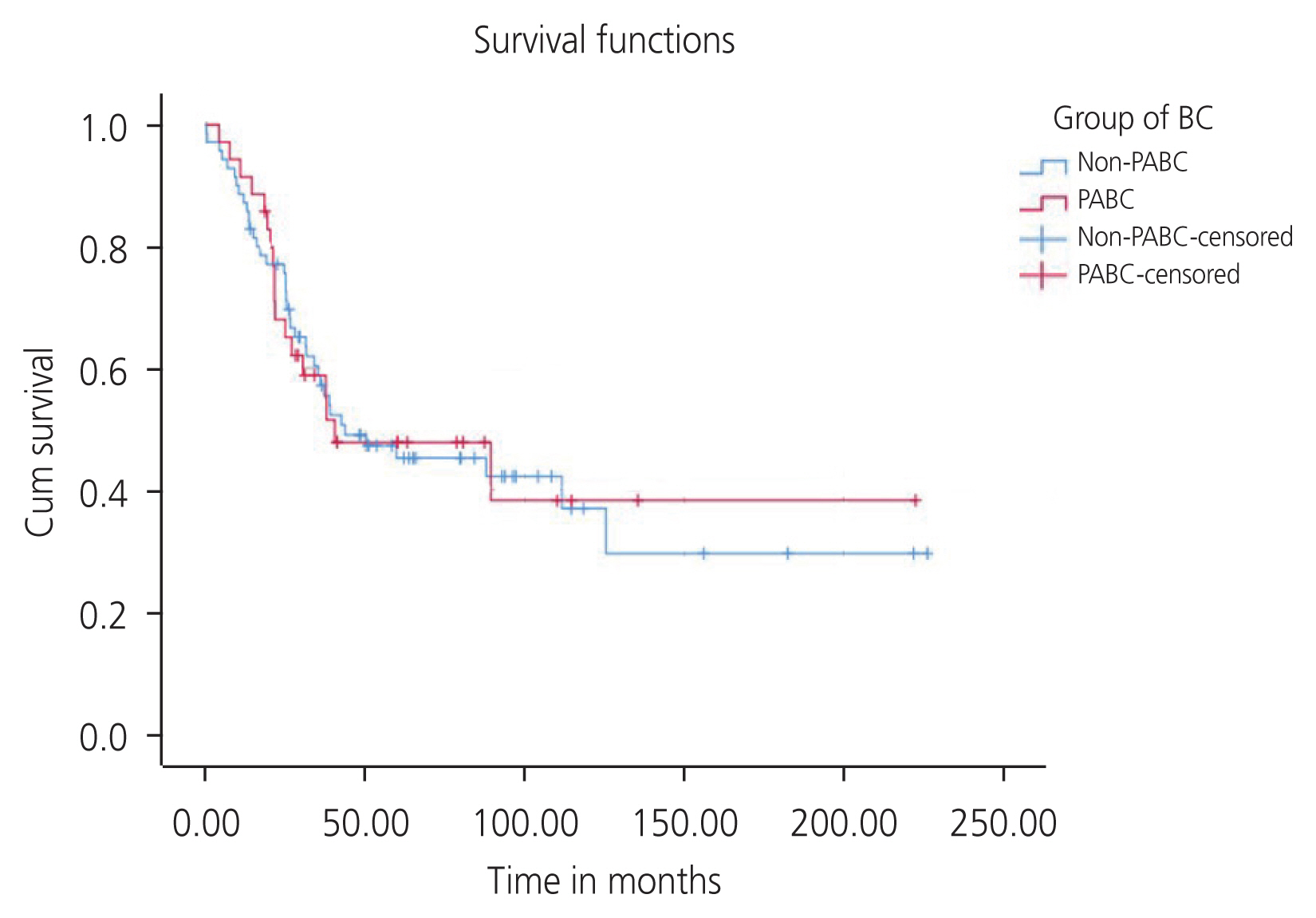
The median OS in the PABC and non-PABC groups were 40.32 months (95% CI, 0.00–94.18) and 43.59 months (95% CI, 0.00–89.92), respectively. However, the difference was not significant (P=0.938). The cumulative 3-year survival rates in the PABC and non-PABC groups were 58.6% and 55.50%, respectively. The cumulative 5-year survival rates in the PABC and non-PABC groups were 47.54% and 45.11%, respectively (Fig. 1). However, the differences in survival between the PABC and non-PABC groups were not significant (P=0.941).
We further stratified data as non-metastatic (stages I, II, and III) and metastatic (stage IV), then compared the 5-year survival rates between the PABC and non-PABC groups (Table 4). The PABC group had a higher 5-year survival rate for non-metastatic disease but a lower rate for metastatic disease than the non-PABC group. However, the differences in survival rates between the groups were non-significant.
3. Pregnancy outcomes in patients with PABC
Among the 35 PABC cases, seven (20.0%), 12 (34.3%), and five (14.3%) patients were diagnosed with breast cancer during the first, second, and third trimesters, respectively, and 22.9% of patients were diagnosed during the post-partum period. More than 50.0% of the patients had normal deliveries. One patient had medical termination, three experienced miscarriages, and four underwent caesarean delivery. Data related to fetal outcomes were available for only 22 (62.8%) of the patients. More than half of the infants were healthy. The median birth weight was 3,055 g (interquartile range 427.50). Appearance, pulse, grimace, activity, and respiration (APGAR) scores were available for only 15 infants. Twelve infants had an APGAR score of 9.0, while three had a score of 10. Only one preterm delivery was documented. There were no reports of fetal death.
4. Prognostic factors of PABC
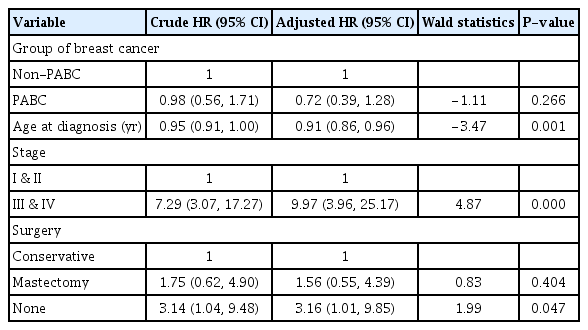
For the univariable analysis, the variables that were found as statistically significant were employment (P=0.021), both sides of cancer (left and right) (P=0.033), III and IV stages of cancer (P<0.001), no surgery (P=0.043), and neoadjuvant chemotherapy (P=0.004). The variables that were found as statistically significant in the multivariate analyses were age, stage of cancer, and history of surgery (Table 5). The aHR for age was 0.91 (95% CI, 0.86–0.96; P=0.001). Patients with advanced stage cancer at diagnosis were 10 times more likely to die compared with those with early stage cancer at diagnosis when adjusted for pregnancy, age, and surgery history. Patients with no history of surgery were three times more likely to die than those with conservative surgery histories (HR, 3.16; 95% CI, 1.01; 9.85; P=0.047). Pregnancy was not a significant independent factor for breast cancer prognosis (HR, 0.72; 95% CI, 0.39–1.28; P=0.266).
Discussion
PABC is rare, but its occurrence is predicted to rise as the average age of marriage and childbirth increases. This study compared the survival outcome of PABC cases with non-PABC cases in Kelantan, Malaysia. Tumor histology of the ductal/lobular type was common in both groups. ER-negative tumors were more prevalent in the pregnant group, similar to the findings of other studies [20,21]. Also consistent with prior studies, the PABC group had a significantly higher rate of PR-negative tumors and a similar rate of human epidermal growth factor receptor 2-positive tumors in both cohorts [6,11,16,18,22].
Out of 35 patients with PABC, the majority (77.2%) were diagnosed during pregnancy, with a maximum (34.3%) amount of diagnoses made during the second trimester. Consistent with many previous studies, the women with PABC in the current study had normal deliveries and gave birth to healthy newborns [23–26]. Only 8.6% of patients in our PABC group had a miscarriage; moreover, only one infant was reported as underweight (1,820 g) at birth, and two infants had fetal complications. One such infant with a birthweight of 3,000 g and an APGAR score of 9.0 was born with congenital pneumonia. The other was a macrosomic baby who was admitted to the neonatal intensive care unit immediately after birth.
The outcomes of the current study reported no significant difference in survival in both the PABC and non-PABC groups. While several studies have reported a poor survival rate in PABC cases, [6–8,10], many studies have found no significant difference between PABC and non-PABC cases in terms of survival [11,13–16,20].
Pregnancy was not related to poor prognosis in patients with breast cancer. This finding has been supported in several previously published studies [3,13,18,25,26].
In contrast, many reports have suggested that pregnancy is a prognostic factor for breast cancer [9,10,15,27,28]. A study that compared 797 PABC cases with 4,177 non-pregnant controls showed a significantly greater death rate in patients with PABC (39.2%) than in the controls (33.4%) (P=0.002). Moreover, pregnancy was associated with a 4.0% higher risk of death in patients with breast cancer [10].
The significant prognostic factors that were found in this study were age, advanced stage of cancer, and history of surgery. Age was also found to be a prognostic factor in another study [26]. In many other studies, the patient’s age at diagnosis has been independently linked to poor prognosis in several types of breast cancer [29–33]. A possible reason for this finding might be the aggressive cancer-related features observed in young patients with breast cancer [29,34,35]. Advanced cancer stage has also been found as a prognostic factor in other studies [10,18,25,27]. Patients with breast cancer who had histories of significant surgeries had poorer survival rates than those who had more conservative surgery histories. This finding is not surprising, as removal of a primary tumor is important for the treatment of breast cancer. Similar findings have been observed in a previous study that investigated survival in patients with and without surgery. In this study, women who refused surgery had a lower 5-year survival than those who opted for surgery. The risk of death due to breast cancer was 2.1 times higher in women who did not have surgery after adjustment for prognostic factors (clinical and treatment-related characteristics) and three times higher when adjusted for age [36].
This is one of the earlier studies to explore and analyze survival outcomes in Malaysian patients with PABC. Since breast cancer is the topmost cancer affecting Malaysian women, research exploring patients with PABC is essential. The current study employed a retrospective cohort design with pregnant and non-pregnant breast cancer cohorts that were followed to compare survival outcomes. A similar design has been applied in other studies [12,24,25,37]. A retrospective study design allowed us to conduct less expensive research in a shorter time period. This design is also appropriate for rare exposure, which requires a long follow up period for the analysis of outcomes. Another strength of this study is the use of multiple Cox regression for the simultaneous examination of all prognostic factors as well as the application of controls for known and unknown confounders.
Nonetheless, there were some limitations of this study. Even though our study was conducted at two tertiary medical centers, we did not have a large sample size. Since PABC is usually diagnosed in younger females (<40 years), it was difficult to obtain exact matches for age in the non-PABC group. A study with a larger sample size of PABC cases from multiple centers across the country is needed to validate the current study’s findings. Furthermore, there was some percentage of missing data for the essential variables. The missing data might have influenced the outcomes that were observed in this study. Since some recruited cases were identified when genetic testing was not widely available, the study did not include information on patients’ genomic profiles.
In conclusion, our results show that the survival rate of women with PABC in Kelantan, Malaysia, was not significantly different than that of the non-PABC group. Due to its retrospective design and limited sample size, the outcomes of this study should be interpreted carefully.
From the current study, it can be concluded that PABC presents with aggressive tumor features, advanced cancer stage, and ER- and PR-negative tumors. As the advanced stage of cancer was a significant prognostic factor in the current analysis, regular screening and detection of breast cancer at an earlier stage might improve the prognosis of patients with PABC. Those who did not have surgery had significantly poor survival compared with those who had conservative surgery. This study can serve as a foundation for future PABC research in Malaysia and encourage better healthcare services for patients with PABC.
Notes
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
The ethical approval for this study was obtained from the USM Ethics Research Committee (USM/JEPeM/22030144) and the Ministry of Health (NMRR ID-22-01076-6HU [IIR]).
Patient consent
None.
Funding information
Nil.
Acknowledgments
The authors would like to thank the Dean and the staff at the School of Medical Sciences, USM, and HRPZ II hospitals, Kota Bharu, for their cooperation and support.