Incidence and survival of gynecologic cancer including cervical, uterine, ovarian, vaginal, vulvar cancer and gestational trophoblastic neoplasia in Korea, 1999–2019: Korea Central Cancer Registry
Article information
Abstract
Objective
To investigate the incidence, trends, and survival rates of all gynecologic cancers using the Korea Central Cancer Registry (KCCR) database from 1999–2019.
Methods
Gynecologic cancer data were obtained from the KCCR database between 1999 and 2019. Age-standardized incidence rates (ASRs), annual percentage changes, and average annual percentage changes (AAPCs) were calculated. The relative survival rate (RSR) was reported by age group, stage, and 6-year period (I: 1999–2005, II: 2006–2012, III: 2013–2019).
Results
The gynecologic cancer ASRs were 26.2 and 24.9 per 100,000 individuals in 1999 and 2019, respectively. Trends of incidence in gynecologic cancer revealed a decrease in cervical cancer and gestational trophoblastic neoplasia (GTN) with AAPCs of −3.4 and −4.3, respectively. Conversely, the incidence of uterine, ovarian, and vulvar cancers increased with AAPCs of 4.7, 2.3, and 2.1, respectively. AAPC for vaginal cancer showed no change. The 5-year survival rate was highest for GTN (90.5%) and lowest for vaginal cancer (56.6%). An increase in age was correlated with poorer survival rates across all gynecologic cancers, excluding vaginal cancer. For all gynecologic cancer types, the prognosis deteriorates with advancing cancer stages. The RSR of uterine cancer improved consistently across all periods. The ovarian cancer RSR improved more in period III than in periods I or II. Additionally, the vulvar cancer RSR improved more in periods II and III than in period I.
Conclusion
In Korea, the incidence of cervical cancer and GTN decreased, whereas the incidence of uterine, ovarian, and vulvar cancer increased from 1999 to 2019. The RSR for uterine, ovarian, and vulvar cancers showed consistent improvements over different periods. Effective screening programs and the adoption of advanced treatments may be necessary to further reduce the burden of gynecologic cancer.
Introduction
Gynecologic cancer refers to malignancies that develop along the gynecologic tract and extend from the ovaries to the vulva. Depending on its location, it can be classified as ovarian, fallopian tube, uterine, cervical, vaginal, or vulvar cancer. Collectively, gynecologic cancers accounted for 15.2% of cancer cases in women and 15.2% of female cancer deaths in 2020 worldwide [1]. According to the 2020 GLOBOCAN report, there were 604,127 new cases and 341,831 deaths from cervical cancer, 417,367 cases and 97,370 deaths from uterine cancer, and 313,959 new cases and 207,252 deaths from ovarian cancer worldwide in 2020 [1]. The cases of vulvar and vaginal cancers were relatively low, accounting for 45,240 and 17,908 cases, respectively, and 17,427 and 7,995 deaths, respectively, in 2020 globally [1].
Cancer is a leading cause of death in Korea [2]. In response, the National Plan for Cancer Control was initiated in 1996 and is still under implementation. A key component of this initiative is the annual statistical report by the Korea Central Cancer Registry (KCCR), which provides cancer registration statistics annually to investigate epidemiologic relationships and promote public health [3]. Estimates of only three major gynecologic cancers-cervical, uterine, and ovarian cancers-are presented in the KCCR statistical report [3].
Uterine cancer can be subdivided into endometrial and non-endometrial cancers, such as uterine sarcoma; however, many studies have primarily focused on the incidence and survival of endometrial cancer patients [4,5]. Recently, fallopian tube and primary peritoneal cancers have been categorized as epithelial ovarian cancer owing to similar treatment approaches and are collectively referred to as primary peritoneal, epithelial ovarian, and fallopian tube (POFT) cancer [6]. Additionally, while gestational trophoblastic neoplasia (GTN) is anatomically classified as a uterine disease, its distinct pregnancy-related pathogenesis warrants separate epidemiological analysis. Only a few epidemiological studies have reported minor cancers, including vaginal and vulvar cancers, thereby necessitating further granular analyses of the incidence and survival of all types of gynecologic cancers and understanding their trends for more effective control and prevention in the future.
Therefore, this study aimed to investigate the incidence, trends, and survival rates of all gynecologic cancers between 1999 and 2019 using the KCCR database.
Materials and methods
1. Data source
Data were sourced from the KCCR, a nationwide population-based cancer registry operated by the Ministry of Health and Welfare in Korea. The KCCR provides comprehensive coverage of the entire Korean population and has collected information on approximately 98.0% of cancer cases in Korea since 1999 [3]. The KCCR collects data on patient age, diagnosis date, primary tumor site, and histology in accordance with the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) [7] and Surveillance, Epidemiology, and End Results (SEER) stages [8], which have been collected since 2005. For this study, the incidence and survival data of gynecologic cancers between 1999 and 2019 were extracted from the KCCR. To estimate the relative survival rate, the survival status of each patient was tracked until December 31, 2020. Mortality data were obtained from Statistics Korea [2]. This study was approved by the Institutional Review Board of the National Cancer Center (NCC2022-0321).
Gynecologic cancers were categorized as cervical cancer (C53), uterine cancer (C54–C55), ovarian cancer (C56, C57, and C48 in females), vaginal cancer (C52), vulvar cancer (C51), and GTN (C58) in accordance with the current ICD-10 scheme [9]. The behavior code (incorporated as the fifth digit in the morphology field in ICD-O) of “/3 (malignant)” was analyzed. In this analysis, fallopian tube, primary peritoneal, and ovarian cancers were collectively considered a single type of ovarian cancer. Histologically, uterine cancers were divided into endometrial (C54.1) and non-endometrial cancers (C54.0, C54.2, C54.3, C54.8, C54.9, and C55). Ovarian cancers were divided into epithelial and non-epithelial cancers using the ICD-O-3 morphology code, following the classification standard presented by Matz et al. [10] (Supplementary Table 1). GTN encodes gestational choriocarcinoma of the uterus. In this study, the period from 1999 to 2019 was divided into periods I (1999–2005), II (2006–2012), and III (2013–2019).
2. Statistical analysis
The incidence represents the age-standardized rate (ASRs) per 100,000 people. The ASR is the weighted average of age-specific rates standardized using Segi’s world standard population [11]. Annual percent change (APC) was calculated based on a linear model using the formula (exp [β]−1) ×100, where β is the slope of the regression of the natural logarithm of ASR in a calendar year. Trends in ASRs were estimated using Joinpoint regression [12]. The average annual percent change (AAPC) is presented as the average APC over multiple years. Relative survival rates (RSRs) were defined as the ratio of the observed survival of patients to the expected survival in the general population, adjusting for the effects of other causes of death using the standard population life table provided by Statistics Korea [2]. The RSRs were estimated using the Ederer II method [13], with minor corrections based on an algorithm devised by Paul Dickman [14]. P<0.05 was considered statistically significant. Statistical analyses were performed using SEER*Stat Joinpoint Regression Program ver. 5.0.2 (National Cancer Institute, Bethesda, MD, USA), and SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
1. Incidence of gynecologic cancer
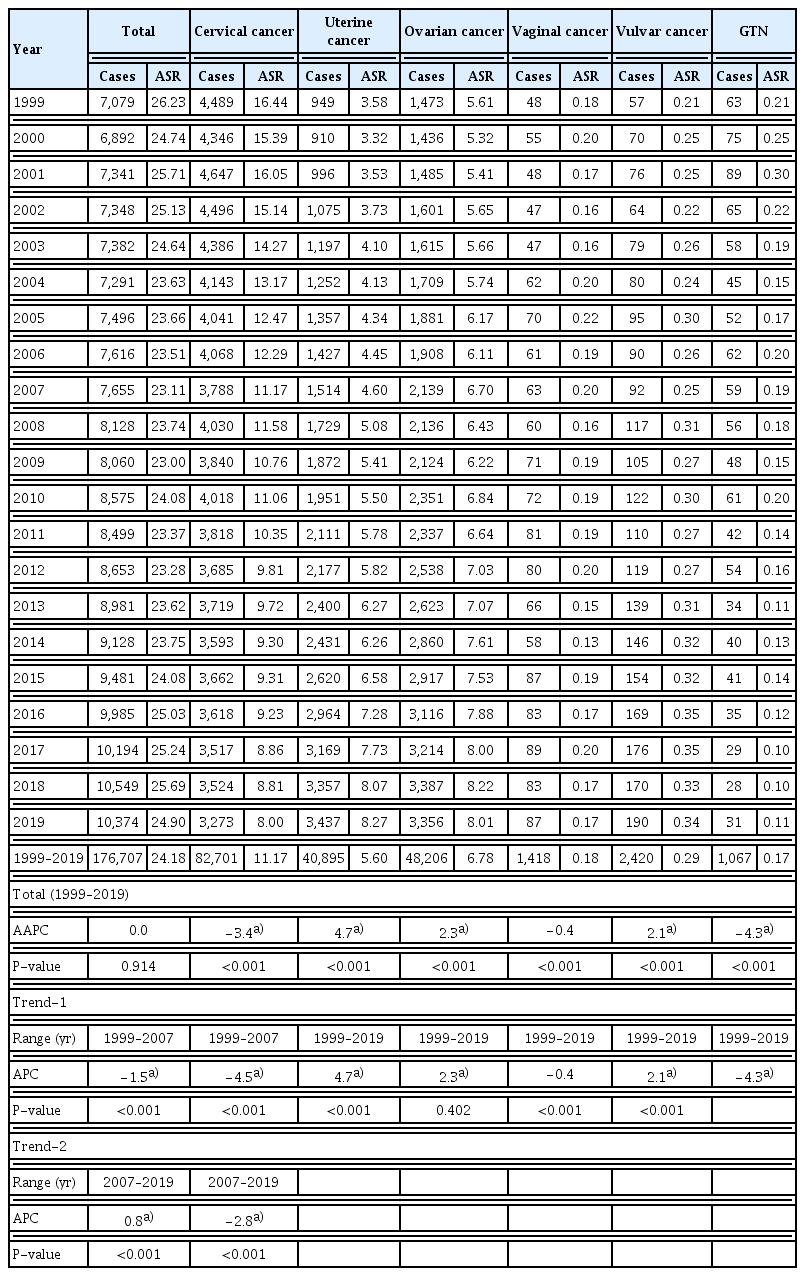
There were 7,079 new cases and 1,818 deaths from gynecologic cancer in 1999 and 10,374 new cases and 2,859 deaths in 2019 (Fig. 1A, Table 1, Supplementary Table 2). Between 1999 and 2019, the ASR of gynecologic cancer incidence did not significantly change, exhibiting an AAPC of 0.0 (P=0.914) (Table 1). However, a shift in the ASR trend was observed. From 1999 to 2007, the ASR of incidence significantly decreased with an APC of −1.5 (P<0.001). In contrast, from 2007 to 2019, ASR significantly increased, with an APC=0.8 (P<0.001) (Table 1).
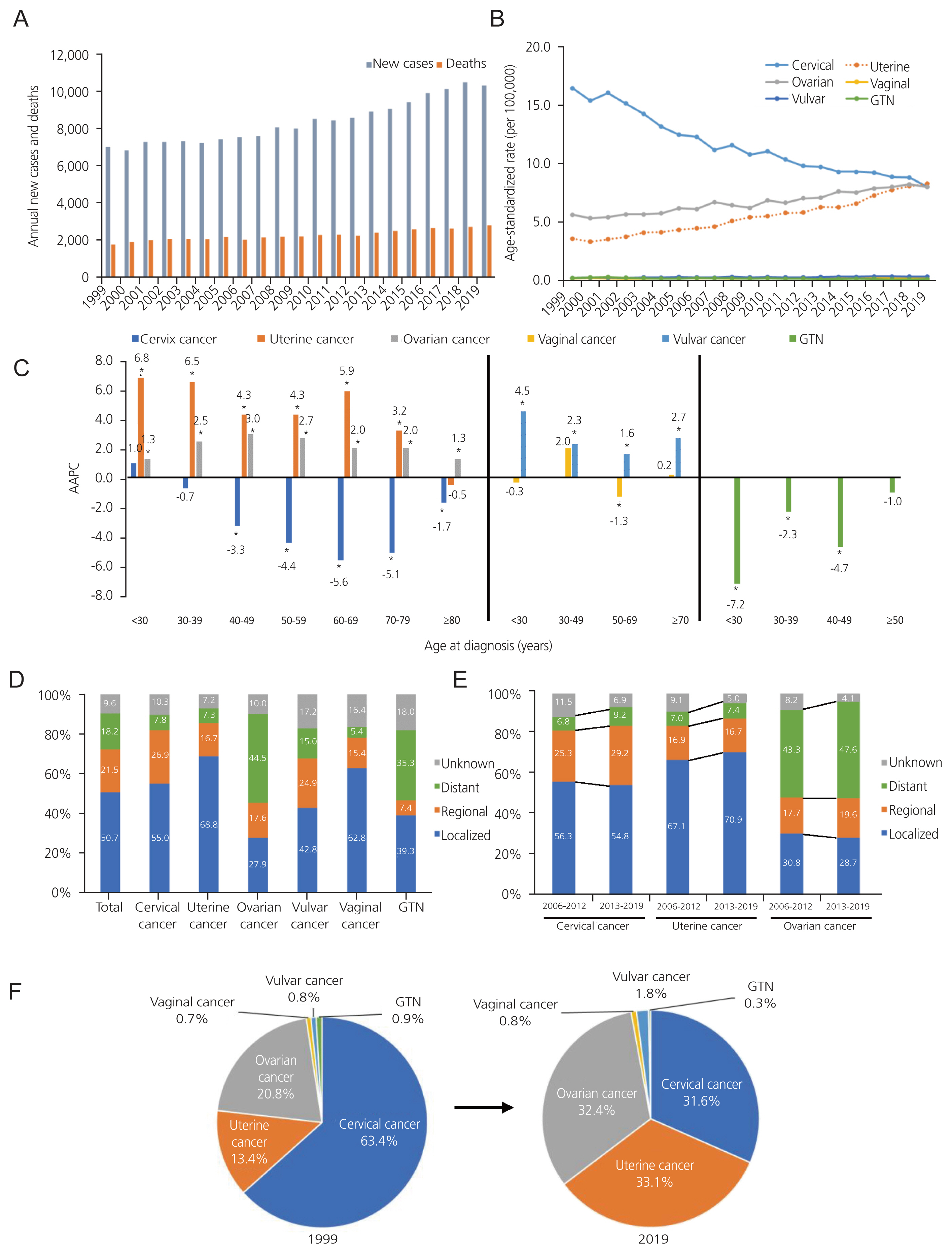
Incidence of gynecologic cancer in Korea, from 1999 to 2019. (A) The annual number of new cases and deaths. (B) The age-standardized rates of incidence by types of gynecologic cancer. (C) Average annual percent changes (AAPC) in the incidence by age group and types of gynecologic cancer. (D) Distribution of SEER stage by types of gynecologic cancer. (E) Secular trends of SEER stage distribution. (F) The percentages of types of gynecologic cancer in 1999 and 2019. *Statistically significant (P<0.05). GTN, gestational trophoblastic neoplasia; SEER, Surveillance, Epidemiology, and End Results.
In 1999, 4,489 new cases of cervical cancer were reported, which decreased to 3,273 in 2019 (Table 1). From 1999 to 2019, ASR of the incidence of cervical cancer significantly decreased, with an AAPC of −3.4 (P<0.001) (Fig. 1B, Table 1). Over the past 21 years, the downward trend in the incidence changed in 2007. From 1999 to 2007, APC was −4.5 (P<0.001), while its decline slowed to −2.8 (P<0.001) from 2007 to 2019 (Table 1). When analyzed based on age group, the incidence significantly decreased across age groups of 40–49, 50–59, 60–69, 70–79, and ≥80 years from 1999 to 2019, with the greatest decrease observed in the 60–69 years age group at an AAPC of −5.6 (P<0.001) (Fig. 1C, Supplementary Table 3). Regarding the distribution of cervical cancer according to the SEER stage, the majority of cases were localized diseases (Fig. 1D). However, the number of uterine and ovarian cancers is increasing annually. The number of new uterine cancer cases was 949 in 1999, an increased 3.6 times from 3,437 in 2019. The ASR of the incidence of uterine cancer significantly increased, as evidenced by an AAPC of 4.7 from 1999 to 2019 (P<0.001) (Fig. 1B, Table 1). When categorized based on age, AAPC significantly increased across all age groups except for those aged 80 and above (Fig. 1C, Supplementary Table 3). The incidence of uterine cancer increased in the <30 and 30–39 years age groups, which had an AAPC of 6.8 (P<0.001) and 6.5 (P<0.001), respectively. The 60–69 years age group also showed a high increase in incidence, with an AAPC of 5.9 (P<0.001) (Fig. 1C, Supplementary Table 3). Regarding the distribution of uterine cancer by SEER stage, localized disease accounted for 68.8% of uterine cancer cases (Fig. 1D).
For ovarian cancer, the number of new cases increased from 1,473 in 1999 to 3,356, a 2.3-fold increase. The ASR of ovarian cancer incidence significantly increased with an AAPC of 2.3 (P<0.001) (Fig. 1B, Table 1). From 1999 to 2019, the incidence of ovarian cancer significantly increased across all age groups, with the most pronounced increase observed in 40–49 years age group at an AAPC of 3.0 (P<0.001) (Fig. 1C, Supplementary Table 3). Considering the distribution of ovarian cancer according to the SEER stage, distant diseases comprised 44.5% of all ovarian cancer cases, unlike the distribution of cervical and uterine cancer cases (Fig. 1D).
Recently, the ranking of the top three gynecologic cancers in Korea, including cervical, uterine, and ovarian cancers, has demonstrated a shift. In 1999, cervical cancer was the most commonly diagnosed gynecologic cancer (63.4% of all cases), followed by ovarian cancer (20.8%) and uterine cancer (13.4%). However, the incidences of these three types of cancers have been similar in recent years. In 2019, uterine cancer was the most commonly diagnosed cancer (33.1% of the total cases), followed by ovarian cancer (32.4%) and cervical cancer (31.6%) (Fig. 1F).
Minor gynecologic cancers include vaginal cancer, vulvar cancer, and GTN. No significant change was observed in the ASR of the incidence of vaginal cancer between 1999 and 2019. There were 87 new vaginal cancer cases in 1999 and 2019, with ASR of 0.18 and 0.17, respectively (Table 1). When broken down by age groups, only the 50–69 years age group for vaginal cancer showed a decline in AAPC at −1.3 (P=0.010) (Fig. 1C, Supplementary Table 3). In contrast, the ASR of vulvar cancer incidence significantly increased with an AAPC of 2.1 (P<0.001) between 1999 and 2019. The number of new vulvar cancer cases has increased from 57 in 1999 to 190 in 2019 (Table 1). Additionally, the incidence of vulvar cancer increased in all age groups (Fig. 1C, Supplementary Table 3). When examined by SEER stage, the majority of cases were localized diseases (Fig. 1D). From 1999 to 2019, the ASR of the incidence of GTN significantly decreased, with an AAPC of −4.3 (P<0.001). There were 63 new cases in 1999, and only 31 cases in 2019 (Table 1). AAPC decreased across all age groups except for individuals aged 50 and above (Fig. 1C, Supplementary Table 3).
2. Survival of gynecologic cancer
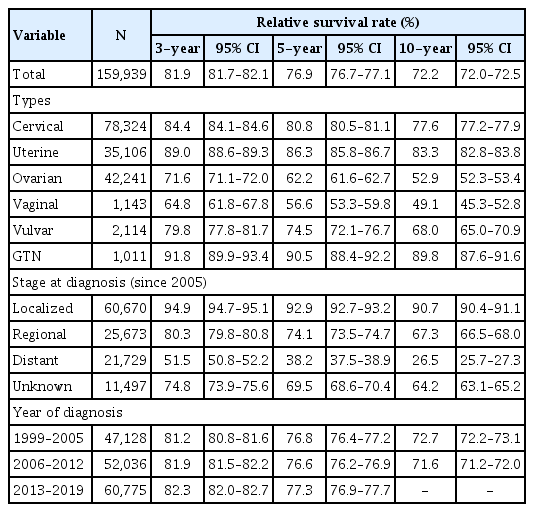
Table 2 displays the overall 3-year, 5-year, and 10-year survival rates of gynecologic cancer, which were 81.9% (95% confidence interval [CI], 81.7% to 82.1%), 76.9% (95% CI, 76.7% to 77.1%) and 72.2% (95% CI, 72.0% to 72.5%), respectively. Comparing the survival rates by the type of gynecologic cancer, the 3-year, 5-year, and 10-year survival rates were notably the best for GTN, followed by uterine cancer, cervical cancer, vulvar cancer, ovarian cancer, and vaginal cancer, in descending order (Fig. 2A). Nonetheless, the 10-year survival rate of the patients with vaginal cancer did not differ significantly from that of the patients with ovarian cancer.

Relative survival rate (RSR) of gynecologic cancer in Korea, from 1999 to 2019. (A) RSR of gynecologic cancer by cancer types. (B) RSR of uterine cancer by histologic type. (C) RSR of ovarian cancer by histologic type.
Among the gynecologic cancer types, uterine cancer was further divided into endometrial and non-endometrial cancers (representative sarcoma), while ovarian cancer was divided into epithelial type (representative POFT), non-epithelial type, and unknown types. The 3-year, 5-year, and 10-year survival rates in patients with endometrial cancer were significantly higher than those in patients without endometrial cancer. The 5-year survival rates of endometrial and non-endometrial cancers were 88.5% (95% CI, 88.0% to 88.9%) and 69.9% (95% CI, 68.3% to 71.3%), respectively (Fig. 2B, Supplementary Table 4). In ovarian cancer, the 3-year, 5-year, and 10-year survival rates were significantly higher in the non-epithelial type and lower in the unknown type. The 5-year survival rates of non-epithelial, epithelial, and unknown type were 86.3% (95% CI, 85.3% to 87.3%), 61.7% (95% CI, 61.1% to 62.3%), and 33.1% (95% CI, 31.5% to 34.7%), respectively (Fig. 2C, Supplementary Table 5).
The 3-year, 5-year, and 10-year survival rates of cervical cancer were 84.4% (95% CI, 84.1% to 84.6%), 80.8% (95% CI, 80.5% to 81.1%) and 77.6% (95% CI, 77.2% to 77.9%), respectively (Supplementary Table 6). When examining trends across age groups, there was a significant decline in the 3-year, 5-year, and 10-year survival rates of cervical cancer as age increased, except in the <30 age group. The 3-year and 5-year survival rates were the highest in the 30–39 age group at 91.8% (95% CI, 91.3% to 92.2%) and 89.4% (95% CI, 88.8% to 89.9%), respectively (Fig. 3A, Supplementary Table 6). When assessed by SEER stage, the 3-year, 5-year, and 10-year survival rates were the highest for localized diseases, followed by regional and distant diseases. For example, the 5-year survival rate were 92.7% (95% CI, 92.3% to 93.0%) for localized, 72.8% (95% CI, 72.0% to 73.7%) for regional, and 27.7% (95% CI, 26.2% to 29.2%) for distant diseases (Fig. 4A, Supplementary Table 6). Survival rates did not differ across the different periods (Fig. 5A). However, the 5-year survival rate of localized diseases from periods II to III significantly increased, with rates improving from 92.0% (95% CI, 91.4% to 92.5%) to 94.0% (95% CI, 93.5% to 94.6%) (Fig. 5B, Supplementary Table 7).
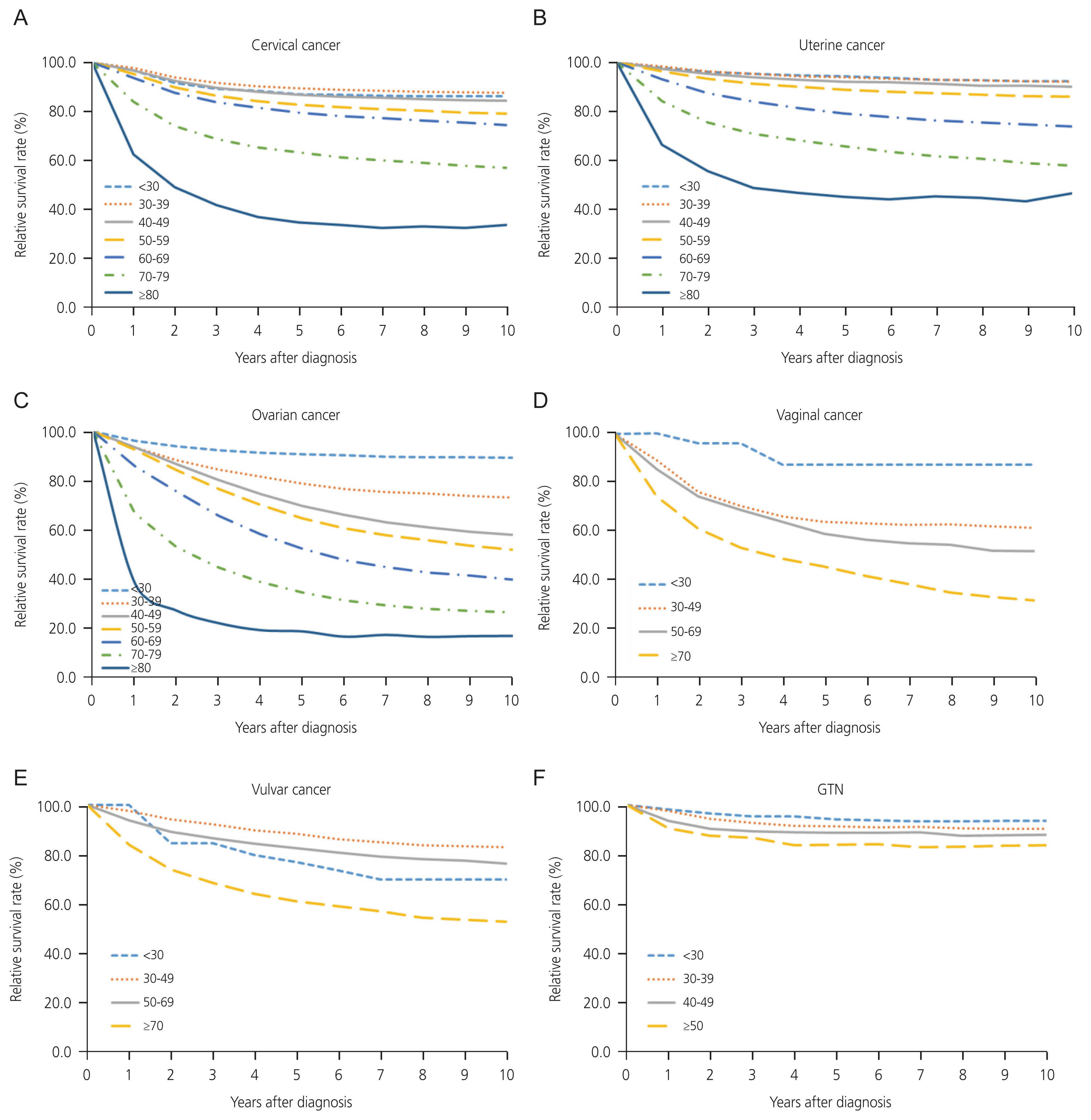
Relative survival rate of gynecologic cancer by age groups in Korea, from 1999 to 2019. (A) Cervical cancer. (B) Uterine cancer. (C) Ovarian cancer. (D) Vaginal cancer. (E) Vulvar cancer. (F) Gestational trophoblastic neoplasia (GTN).
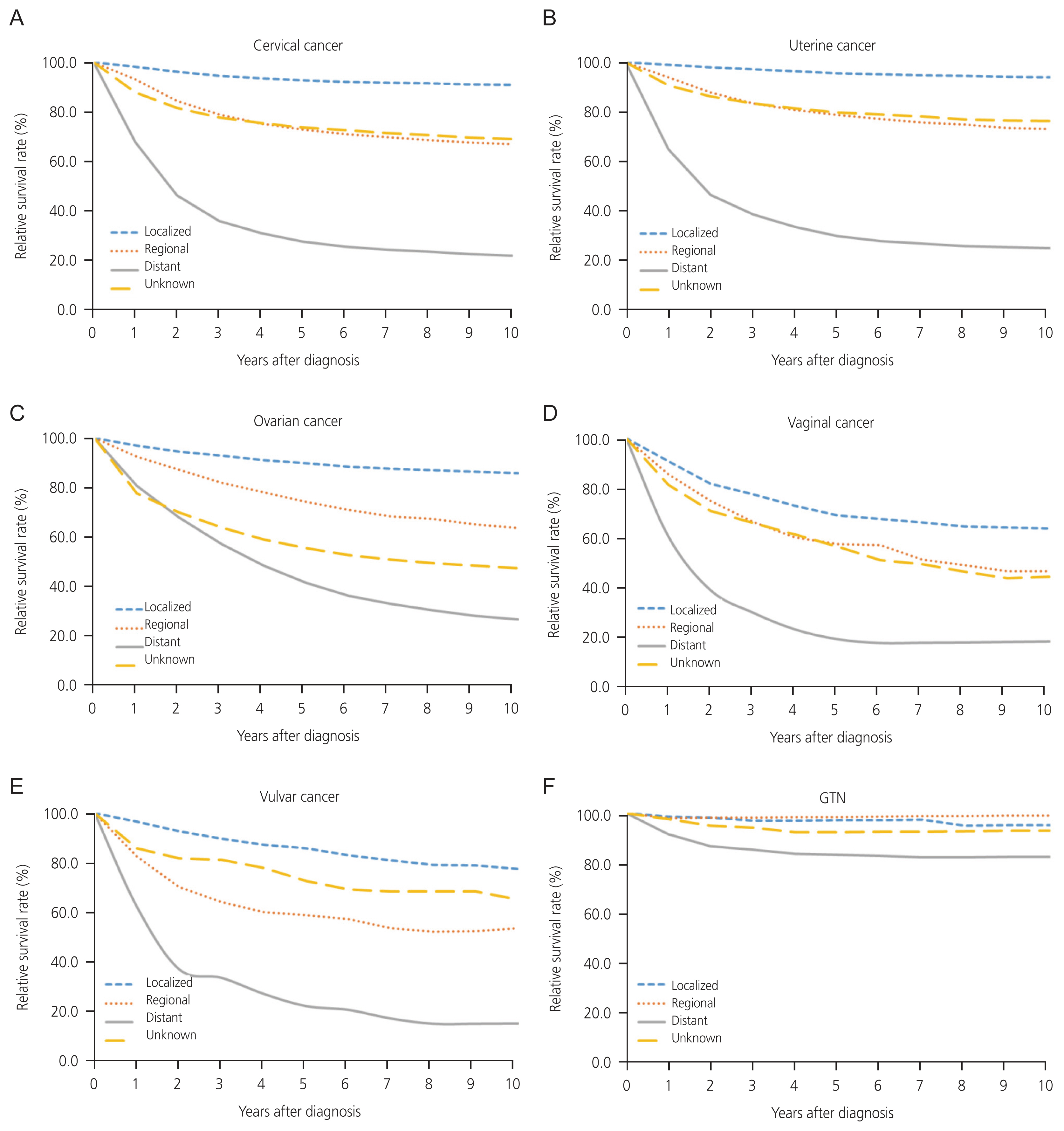
Relative survival rate of gynecologic cancer by SEER stage in Korea by, from 2006 to 2019. (A) Cervical cancer. (B) Uterine cancer. (C) Ovarian cancer. (D) Vaginal cancer. (E) Vulvar cancer. (F) Gestational trophoblastic neoplasia (GTN). SEER, Surveillance, Epidemiology, and End Results.

The trend of 5-year survival rate of gynecologic cancer by year of diagnosis in Korea, from 1999 to 2019. (A) The trend of 5-year survival rate by year of diagnosis. (B) The trend of 5-year survival rate of cervical, uterine, and ovarian cancer by SEER stage between period 2006–2012 and 2013–2019. *Statistically significant (P<0.05). GTN, gestational trophoblastic neoplasia; SEER, Surveillance, Epidemiology, and End Results.
The 3-year, 5-year, and 10-year survival rates of uterine cancer were 89.0% (95% CI, 88.6% to 89.3%), 86.3% (95% CI, 85.8% to 86.7%), and 83.3% (95% CI, 82.8% to 83.8%), respectively (Supplementary Table 4). The 5-year survival rate of uterine cancer significantly decreased as age increased for age groups 30 years and older, while the age groups of <30 and 30–39 showed similar survival rates. The 10-year survival rate significantly decreased with age in age groups 50s and older. However, the 10-year survival rate for patients with uterine cancer was higher than 90.0% for those aged <50 years (Fig. 3B, Supplementary Table 4). When examined by SEER stage, the survival rates were highest for localized diseases, followed by regional diseases, and were lowest for distant diseases (Fig. 4B, Supplementary Table 4). The 3-year, 5-year, and 10-year survival rates improved across all periods (Fig. 5A, Supplementary Table 4).
The 3-year, 5-year, and 10-year survival rates of ovarian cancer were 71.6% (95% CI, 71.1% to 72.0%), 62.2% (95% CI, 61.6% to 62.7%) and 52.9% (95% CI, 52.3% to 53.4%), respectively (Supplementary Table 5). The overall survival rate of patients with ovarian cancer significantly decreased with increasing age (Fig. 3C, Supplementary Table 5). Considering the SEER stage, the survival rate was the highest for localized diseases, followed by regional diseases, and the lowest for distant diseases (Fig. 4C, Supplementary Table 5). The 3-year survival rate showed improvement from period I to III, while the 5-year survival rate of period III (64.1%; 95% CI, 63.2% to 64.9%) was significantly higher than period I or II (60.4%; 95% CI, 59.4% to 61.4% or 61.3%; 95% CI, 60.4% to 62.1%) (Fig. 5A, Supplementary Table 5). Additionally, the 5-year survival rate from Periods II to III for localized, regional, and distant types of ovarian cancer significantly increased (Fig. 5B, Supplementary Table 7).
In minor cancers, there was no significant difference in survival rates across age groups (Fig. 3D–F). For vaginal cancer, the 5-year survival rate for distant disease was significantly lower than that for localized or regional disease, whereas there was no difference between localized and regional disease (Fig. 4D, Supplementary Table 8). For vulvar cancer, the survival rates were highest for localized diseases, followed by regional diseases, and were lowest for distant diseases (Fig. 4E, Supplementary Table 9). The 5-year survival rate of patients with vulvar cancer significantly improved from periods I to II (Fig. 5A, Supplementary Table 9). For GTN, the 5-year survival rate of distant disease was significantly lower than that of localized disease; however, distant disease exhibited a survival rate higher than 82.5% (Fig. 4F, Supplementary Table 10).
Discussion
Most studies primarily report the incidence and survival rates of the three major gynecologic cancers, cervical, endometrial, and ovarian cancers, because they account for the high incidence of gynecologic cancers. However, the paucity of comprehensive epidemiological information on minor, less common gynecologic cancers has complicated the representation of the entire scope of gynecologic cancers by focusing solely on the top three cancer types. In response, this study identified the incidence, trends, and survival of all gynecologic cancer types, including cervical, uterine, ovarian, vaginal, and vulvar cancer, and GTN, over 21 years in Korea.
In Korea, from 1999 to 2019, the incidence of gynecologic cancer remained unchanged with an AAPC of 0.0. This stability reflects a decrease in the incidence of cervical cancer and an increase in the incidence of uterine and ovarian cancers. Although minor cancers account for approximately 2–3% of gynecologic cancers, patterns have been identified from 1999 to 2019, vulvar cancer incidence increased, GTN decreased, and no change was found in vaginal cancer. In 2007, the ASR trend showed a notable shift for all gynecologic cancers when the incidence began to rise after a decline. This change appears to correlate with a significant drop in cervical cancer cases between 1999 and 2007, marked by an APC of −4.5, which later slowed slightly to −2.8, despite the gradual increase in uterine and ovarian cancer cases from 2007 to 2019. The launch of cervical cancer screening projects to promote public health in 1999, which persists today, likely influenced the observed decrease in the incidence of cervical cancer. Historically, cervical cancer constituted approximately 60.0% of all gynecologic cancers. However, the three primary gynecologic cancer types account for approximately 30.0% of the total.
The incidence of cervical cancer decreased across the age groups of 40–49, 50–59, 60–69, 70–79, and >80 years. The key risk factor for cervical cancer is human papillomavirus (HPV) infection, after which precancerous lesions slowly progress to cervical cancer in 10–20 years [15,16]. With the introduction of the National Cervical Screening Program (NCSP) in Korea in 1999, which utilizes cervical cytology, there has been an uptick in the detection of precancerous lesions, enabling early treatment [17]. Considering the progression timeline of precancerous lesions and the age at which individuals reach adulthood since 1999, it can be inferred that the NCSP helped reduce the number of cervical cancer cases in individuals aged 40 years or older. Nonetheless, the results showed no decline in incidence in young women in Korea. These trends in Korea are similar to those reported in Norway and other European countries, where despite an overall 68.0% reduction in cervical cancer incidence, the incidence of cervical cancer has increased among women under 30 years of age [18,19].
The survival rate of patients with cervical cancer decreases with age. However, the 5-year survival rate for the 30–39 age group was higher than that for the <30 age group. A study from the United Kingdom (UK) showed that cervical cancer in young women (<25 years) tended to be more aggressive and advanced at the time of diagnosis (stage 1B+or worse), and a higher proportion of younger women were diagnosed with adenosquamous carcinoma and other rare histological types [19]. The treatment paradigm for cervical cancer has not changed significantly over the past two decades, showing no difference in overall survival by period. Nonetheless, the 5-year survival rate of patients with localized disease significantly improved from 92.0% to 94.0% in periods II and III.
In 2019, the incidence of uterine cancer was the highest among all gynecologic cancers, with an ASR of 8.27 per 100,000, marking a 3.6-fold increase from 1999 to 2019. According to Global Cancer Statistics 2020, the ASR for the corpus uteri is 8.2 in Eastern Asia, which is consistent with our findings. In contrast, North America reported the highest ASR of 21.1, whereas Middle Africa reported the lowest of 2.3 [1]. The increase in ASR is thought to be primarily driven by risk factors associated with endometrial cancer, given that it constituted 88.4% of the uterine cancers in this study, despite the known differences in risk factors and prognoses between endometrial cancer and sarcoma. Of 127 meta-analyses that included cohort studies, body mass index (BMI), waist-to-hip ratio, and nulliparity were associated with increased endometrial cancer risk [20]. Additionally, a substantial population-based cohort study of Korean women reported metabolic syndrome as another risk factor [21]. The prevalence of metabolic syndrome increased among adolescents in Korea between 2007 and 2018, with changes in dietary habits and physical inactivity being the contributing factors [22]. Additionally, Korea’s total fertility rate (TFR) was only 0.78 in 2022 [23], placing Korea among the ultralow-fertility countries, in contrast to the TFR of 1.63 in Organization for Economic Co-operation and Development countries [24]. The increase in obesity combined with the decrease in birth rates may have contributed to the pronounced increase in the ASR incidence of uterine cancer in the <30 and 30–39 age groups.
With a 10-year survival rate >83.0%, uterine cancer has the highest survival rate among gynecologic cancers, excluding GTN. The most important prognostic factor for endometrial cancer is its stage, and in this study, localized diseases accounted for 58.3% of all uterine cancer cases. Early symptoms such as amenorrhea or abnormal uterine bleeding, especially in endometrioid endometrial cancer cases, combined with the development of sonography or examinations for high-risk groups, may also aid in early cancer detection. However, it is worth noting that there is no established effective screening program for uterine cancer in clinical practice. Among gynecologic cancers, only uterine cancer showed continuous improvement in the 3-year, 5-year, and 10-year survival rates across the three periods of 1999–2005, 2006–2012, and 2013–2019, respectively. While the incidence of regional and distant diseases remained unchanged from periods II to III, an increase in localized propositions may have contributed to the improvement in survival rates (Fig. 1E). Additionally, when examined by SEER stage (since 2006), the regional stage from 2013 to 2019 showed an improved 5-year survival rate compared with that from 2006 to 2012. This improvement can be attributed to multiple factors. First, more patients were given an accurate diagnosis and early detection than in the first period. Second, the late 2000s saw the introduction of the concept of routine lymphadenectomy, encompassing the para-aortic region and linked with higher survival rates [25]. Lastly, the shift from radiotherapy to chemotherapy for adjuvant therapy after initial surgery, as well as the shift in regimen from anthracyclines and platinum-based drugs to taxane and platinum for those at an advanced stage or with high risk, may also be contributing factors [26,27]. A similar improvement in the survival rate was also observed in a 40-year population-based analysis in Japan, which reported significantly improved prognoses for localized and regional uterine cancer between 2000 and 2016 compared to 1977 and 2000 [28].
Similar to uterine cancer, the number of ovarian cancers steadily increased by approximately 2.3-folds. The incidence of ovarian cancers increased across all age groups, with the highest increase observed in the 40–49-year group. A report from 2 years ago indicated a high rise in incidence between 70–79 and >80 years of age for POFT cancer cases [5]. This finding has an effect, although minor, on this study through the inclusion of 12.3% of non-epithelial types, which predominantly manifest in women under 40 years of age [29]. The 5-year survival rate of ovarian cancer is very low (62.2%), largely because the epithelial type accounts for 78.3% of all ovarian cancer cases. The RSR of the unknown type was the lowest in ovarian cancer, suggesting that most of them might include advanced-stage cases treated with neoadjuvant chemotherapy, in which only malignant cells have been identified by ascites cytology. Known risk factors for ovarian cancer include a family history of ovarian, breast, uterine, or colorectal cancer; genetic mutations such as breast cancer susceptibility gene (BRCA); menopause; age; obesity; and a higher BMI [30]. Despite these known risks, there is still no screening program for early detection, which leads to the diagnosis of 62.1% of patients at an advanced stage. Fortunately, the survival rate of patients with ovarian cancer has improved over time. From periods I to III, the 3-year survival rate improved significantly. Moreover, the 5-year survival rate in period III surpassed that in periods I and II, which may be related to efforts to ensure no residual disease (RD) after surgery as well as the revolutionary introduction of targeted therapy, although the proportion of localized disease tended to decrease. No gross RD was associated with improved overall survival, and radical surgery was effective in achieving no gross RD [31]. With advances in surgery and collaborations with other departments, debulking surgery has become increasingly feasible. Additionally, the 2007 approval of bevacizumab in Korea in 2007 has improved the progression-free survival of women with ovarian cancer. The benefits of this drug regarding both progression-free and overall survival rates were greater among those at a high risk of disease progression [32]. Furthermore, maintenance therapy with PARP inhibitors, approved in Korea in 2015, substantially enhanced progression-free survival among women with newly diagnosed advanced ovarian cancer and a BRCA1/2 mutation, resulting in a 70.0% lower risk of disease progression or death [33].
The ASR of minor cancers remains relatively low, ranging from approximately 0.1 to 0.4 per 100,000 individuals for each type. Approximately 78.0% of vaginal cancers and 24.0% of vulvar cancers are attributable to HPV infection [34]. Screening for these cancers is not cost-effective, and optimal screening strategies are yet to be developed. Based on this study, vulvar cancer survival rates improved from 1995 to 2005 to 2006 to 2012, a trend also observed in studies from the UK and Norway, which reported improved survival rates in recent years [35,36]. The ASR of vaginal cancer was 0.18 per 100,000, and the 5-year survival rate was the worst among gynecologic cancers at 56.6%. A French vaginal cancer study using data spanning from 1990 to 2018 mirrored these findings, presenting an ASR of incidence of 0.2 and concluding that age-standardized net survival at 1 and 5 years after diagnosis remains low at 74.0% and 45.0%, respectively [37]. The number of GTN cases has decreased over the past 21 years, with only approximately 30 recent annual cases being reported. The currently available chemotherapy regimens have drastically changed to achieve nearly 100.0% cure rates [38], which is reflected in this study by the impressive 5-year survival rate of 90.5% for GTN.
From 1999 to 2019, there was a decline in the incidence of cervical cancer and GTN in Korea, whereas the incidence of uterine, ovarian, and vulvar cancers increased. Despite these shifts, the overall incidence of gynecologic cancers remains relatively unchanged. The 3-year, 5-year, and 10-year survival rates for gynecologic cancers were 81.9%, 76.9%, and 72.2%, respectively. When comparing the survival rates of patients with gynecologic cancer, those with GTN exhibited the highest survival rate, followed by those with uterine, cervical, vulvar, ovarian, and vaginal cancer. Notably, there was an improvement in the RSR of uterine cancer across all periods, and ovarian cancer also showed improved survival after 2013 compared to previous periods. Furthermore, the RSR of vulvar cancer in periods II and III improved compared to that in period I. Such improvements, especially in the survival of patients with ovarian cancer, may offer much-needed mental support to those newly diagnosed or at high risk given their historically poor prognosis. The burden of gynecologic cancer may be reduced by developing effective screening programs and introducing advanced treatments.
Supplementary Information
Acknowledgement
The authors thank Grace J. Lee for revising and refining this manuscript.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
This study was approved by the Institutional Review Boards of National Cancer Center (IRB No. NCC2022-0321), performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
Written informed consent and the use of images from patients are not required for the publication.
Funding information
No fund was provided.