 |
 |
- Search
| Obstet Gynecol Sci > Volume 66(6); 2023 > Article |
|
Abstract
Objective
Regular users of hormonal contraceptive pills show marked heterogeneity in metabolic effects with variations in compositions. This might be due to choice of outcome variables for comparison. Total cholesterol-high-density lipoprotein ratio (TC/HDL) discordance with low-density lipoprotein (LDL-C) has now become an established marker of future risk for atherosclerotic cardiovascular disease and stable to variations in user.
Methods
The present study was a randomized controlled trial to compare prevalence of TC/HDL and LDL discordance among non-obese women with polycystic ovarian syndrome (PCOS) treated with hormonal pills. Women were randomized into three arms, two arms received ultra-low dose pills (Ethinylestradiol [EE] 20 μg with drosperinone 3 mg or EE 15 μg with gestodene 60 μg) and one arm received low dose pill (EE 30 μg with desogestrel 150 μg). The role of baseline participant features and pill composition on discordance was determined.
Results
Discordance was observed in more than a quarter of the participants before intervention. After 1 year of treatment, less than a fifth of the participants were discordant. Ultralow-dose pill users had lower discordance, LDL, and TC than low-dose pill users after 1 year of treatment. The random forest, a non-linear classifier, showed the highest accuracy in predicting discordance. The baseline Parameters with the maximal impact on the occurrence of discordance were triglyceride, homeostatic model assessment for insulin resistance, body mass index, and high density lipoprotein.
Low-dose hormonal pills containing Ethinylestradiol (EE) 30-35 μg combined with second or third-generation progestins are commonly used for cycle control and correction of hirsutism in polycystic ovary syndrome (PCOS) [1]. Its regular use is associated with dyslipidemia, metabolic syndrome (MetS), and risk of cardiovascular events in women with preexisting metabolic disorders [2,3].
A meta-analysis of studies evaluating the effect of low-dose pills on lipid indices in adult premenopausal women showed marked heterogeneity in outcomes. The authors were unable to draw a conclusion on whether “differences in lipid indices among users of different hormonal pills lead to a change in the future risk of atherosclerotic cardiovascular disease (ASCVD)” [4,5]. In recent years, the use of ultra-low-dose hormonal pills containing EE-20 μg or less has become common among women using pills for cycle control in PCOS. In healthy premenopausal women, these pills reduce body mass index (BMI), blood pressure, insulin resistance (IR), and dyslipidemia [6,7]. However, not all studies have been unanimous regarding the better metabolic impact of ultra-low-dose pills on PCOS [8]. Obese women with PCOS on pills containing EE-20 combined with non-androgenic progestins show an increase in both small and large low-density lipoprotein (LDL) particles, free androgen index, and postprandial glucose-insulin ratio [9,10]. The reason for the lack of a predictable pattern in the metabolic effects of the pills across studies was the pathophysiology of dyslipidemia, which did not follow a simple cause-and-effect relationship [11]. The observed effects were modified by BMI, androgen levels, and IR. These parameters also showed ethnic variations, such as the prevalence of obesity being less common and hyperandrogenism (HA), IR, and elevated LDL being more common in Asian women than in women with PCOS of Caucasian or Mediterranean background [12,13].
In comparing the metabolic effects of the pills, the choice of outcome variable was not uniform across the studies. Most of the studies observed changes in serum lipoproteins, IR, or free androgen index to detect variations in the effects. These variables lacked sensitivity and often did not project significant differences owing to high baseline levels in obese women and too small a difference in non-obese women [14,15]. Newer novel markers, such as follistatin and high-sensitivity C-reactive protein, are now under evaluation for the prediction of future ASCVD following therapy with hormonal pills in PCOS [16]. Discordance analysis is an emerging concept for predicting adverse cardiovascular events. It is defined as the percentile value of total cholesterol (TC)/high-density lipoprotein (HDL) for an individual being greater, and the percentile value of LDL or apoB for the same participant being less than the respective population median [17].
Approximately 30.0-70.0% of women diagnosed with PCOS are non-obese, and few studies have reported changes in lipid indices in these women. Women with lean PCOS have lower HDL and HDL2 levels and a higher prevalence of MetS than women with normal ovarian function [18,19]. Currently, no data are available on TC/HDL and LDL discordance in lean women with PCOS. This study aimed to determine whether the choice between ultralow-dose and low-dose hormonal pills affects TC/HDL discordance in non-obese women with PCOS. The null hypothesis posits that there are no significant TC/HDL discordance differences after 1 year of regular hormonal pill use, regardless of pill type. This study also intends to construct a machine-learning model capable of predicting TC/HDL cholesterol discordance based on the baseline attributes of non-obese women with PCOS who consistently use hormonal pills for a year.
This study was a double-blind, randomized, controlled, three-arm trial comparing ultra-low-dose and low-dose hormonal pills. It was performed at a postgraduate teaching institute in West Bengal, India, which serves a low- to middle-income-earning urban population, according to the Indian standard. The study was conducted from December 1, 2019, to November 30, 2022. Women of reproductive age diagnosed with PCOS were included in this study. PCOS was diagnosed according to the 2003 Rotterdam criteria (Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group, 2004). Women who were smokers, with BMI ≥25, hypertension (blood pressure ≥140/90 or on antihypertensive medications), active liver disease, undiagnosed vaginal bleeding, known coagulation disorders, or history of intake of combined oral contraceptive pills in the preceding 3 months and amenorrhea for ≥1 year were excluded. Women were enrolled after screening for attitudes toward and compliance with the study protocol. Written informed consent was obtained from all participants before inclusion and ethical approval was obtained from the Institutional Ethics Committee. This study was prospectively registered with the Clinical Trials Registry of India (CTRI/2018/02/011920).
The study had two intervention arms, where selected participants received ultra-low-dose EE (20 μg) and Drosperinone (3 mg) (Dronis 20; Sun Pharmaceutical Industries Ltd., Mumbai, India) (henceforth designated as EE-20) or EE (15 μg) and Gestodene (60 μg) (Minesse; Pfizer India limited, Mumbai, India) (henceforth designated as EE-15). Participants in the control arm received low dose pill EE (30 μg) and Desogestrel (150 μg) (Novelon; Organon India Ltd., Mumbai, India) (henceforth designated as EE-30). The ultralow-dose pill packs consisted of 24 tablets and four placebos. The control arm consisted of 21 tablets and seven placebos for each cycle. Participants started taking pills on the first day of spontaneous or progestogen-induced menstrual cycle. The treatment regimen was continued for twelve consecutive cycles. All participants were provided written information on the common side effects of hormonal pills, and the protocol followed was the same as that used for contraception.
Participants underwent detailed interviews, clinical evaluations, and investigations before the intervention. The clinical parameters studied were age, body weight, height, and presence of hirsutism (a marker of HA). Hirsutism scores were calculated using the simplified Ferriman-Galwey (sFG) score [20]. Women with sFG score ≥3 were considered to have hirsutism. On the 2 or 3 day of a spontaneous or progestogen-induced cycle, fasting blood glucose, fasting insulin (FI), free T4, thyroid-stimulating hormone (TSH), lipid profile (TC, triglycerides [TG], HDL, LDL and very low-density lipoprotein [VLDL]), and free testosterone assays were performed. Fasting glucose levels were measured by per-oxidase (POD) method. FI, free T4, and TSH were estimated using an automated chemiluminescent immunoassay. The lipid fraction TC was measured using choesterol-oxidase and per-oxidase method (CHOD-POD), HDL was measured using homogenous enzymatic colorimetric assays, and TG was measured using the glycerol 3 phosphate-dihydroacetone phosphate-peroxidase method. LDL levels were calculated using Friedewald’s formula. Assays were performed using a Konelab 20 clinical chemistry analyzer (Thermo Scientific., Waltham, MA, USA). The free testosterone assay was performed using a commercially available free testosterone Elisa kit (Calbiotech, Inc., El Cajon, CA, USA). Abdominal ultrasonography was performed in all patients to study ovarian morphology. The participants were clinically re-evaluated every 3 months or earlier, if necessary. Clinical and blood parameters were measured 1-year post intervention in the same laboratory using the same methods.
The primary outcome was the difference in the proportion of TC/HDL and LDL discordance between the arms after 1 year of treatment. Percentile values of the TC/HDL and LDL ratios were determined, and arm means were calculated. Discordant data points were determined by calculating the difference in individual data points from arm means for both variables and were defined as a positive value for TC/HDL and a negative value for LDL (method described by Elshazly et al. [21]). One-year comparisons between other metabolic and endocrine indices, viz. lipid profile, homeostatic model assessment for insulin resistance (HOMA-IR), and free T, were also performed between the treatment arms. Significant differences in features between discordant and non-discordant participants at baseline and at 1 year were also determined.
Based on the prevalence of PCOS in the study population, we expected to recruit approximately 200-210 participants within the study duration. Therefore, 70 strips for each drug were acquired from the pharmacy. The drugs to be dispensed were pre-packed in identical containers and numbered according to a computer-generated random allocation sequence containing numbers from 1 to 210. Participants were allocated to one of the two intervention arms or the control arm using a software-generated random allocation sequence. Allocation of participants was performed by the process of minimization using minim software so that the arms were similar at baseline. Minimization was done for the following variables: age, BMI, and sFG score. Each participant received an opaque sealed envelope containing a random number between 1 and 210 which corresponded to a numbered pre-packed container. A container was provided to the participants only after completion of the baseline investigations. Random sequence generation, allocation, and drug dispensation were performed by an independent statistician with the help of a resident doctor and a staff nurse. Both the participants and investigators were blinded to the type of intervention drug received until the data analysis was completed.
For comparison between treatment arms, data distribution in the respective arms was examined for normality using the Shapiro-Wilk test. Participant characteristics were compared within arms using a paired t-test for normally distributed data or the Wilcoxon signed-rank test when the distribution was non-normal for continuous variables. Fisher’s exact test was used to analyze categorical variables. Participant characteristics were compared between the arms at baseline and at 1 year using analysis of variance or the Kruskal-Wallis test according to the type of distribution. For any observed differences between the arms, post hoc Tukey’s test was performed to detect differences between pairs. The chi-square test was performed to detect variations in the case of categorical variables, and differences between pairs were determined using Fischer’s extract test (at baseline and at 1 year). A P-value of ≤0.01 was considered statistically significant for all analyses. Comparisons between participant characteristics among discordant and non-discordant participants at baseline and at 1 year were performed using the t-test or Mann-Whitney U-test for continuous variables or the chi-square test for categorical variables.
To predict discordance at 1 year, different classifiers were trained with baseline participant features and information on the type of drug used. These features were selected based on previous studies and their biological plausibility. The data were randomly divided into a training set (comprising 80.0% of the participants) and a validation set (comprising 20.0% of the participants). Ten-fold cross-validation was performed during the training of the classifiers, and the results reported were an average of the 10 runs. Initially, different linear and nonlinear classifiers were trained. The performance parameters of the classifiers were determined and compared. The classifier with the best performance was used to predict discordance. The degree of importance of each predictor is expressed as its Gini index. All experiments reported in this paper were carried out using Orange 3.0, version 3.26.0 (Bioinformatics Lab, Kongresni trg, Ljubljana, Slovenia), machine learning, and a data mining toolkit.
A total of 3,240 women were assessed, of whom 212 fulfilled the diagnostic criteria for PCOS. One hundred and sixty three women were randomized into three arms with 54 women in each intervention arm and 55 women in the control arm. Two women from the control arm did not comply with the study protocol and two were lost to follow-up. Three women from each intervention arm were lost to follow-up. These 10 participants were excluded, and there were 153 participants in the final analysis, with 51 participants in each arm. Fig. 1 depicts the flow of participants during the study period.
The selected cohort had a mean age of 25.02±4.03 years, BMI of 22.59±1.63, sFG score of 2 and mean, free T4 level of 1.68±1.09 ng/dL at baseline. The participants were comparable and similar proportions had discordance in each arm at baseline. Overall, discordance was observed among the 42 participants. Table 1 describes the characteristics of the three treatment arms at baseline and at 1 year. Comparisons were performed within the arms (after the intervention) and between the arms (before and after the intervention). Within-arm comparisons between the baseline and 1-year values showed the following: 1) for the EE-20 arm (at 1 year), BMI increased, and sFG, TSH, free testosterone, and HOMA-IR decreased. Lipid levels did not show any significant changes. The proportion of discordance decreases after treatment. 2) For the EE-15 arm (at 1 year), BMI increased, and sFG, TSH, free testosterone, and HOMA-IR decreased. There was reduction in LDL and increase in HDL the proportion of discordance did not show significant change after treatment. And 3) in the EE-30 arm (at 1 year), BMI increased and sFG, TSH, and free testosterone reduced. HOMA-IR, LDL and TC increased. The proportion of discordance did not change significantly after treatment.
Between-arm comparisons showed that the mean TSH was higher in EE-30 than in EE-20. Free T was higher in both EE-20 and EE-15 than in EE-30. The mean TC and LDL were higher in EE-30 than in EE-20 and EE-15. Overall, discordance was observed in 27 participants at 1 year. The proportion of discordant participants was higher in the EE-30 arm than that in the EE-20 arm.
Table 2 compares the characteristics of the discordant and non-discordant participants at baseline and after the intervention. At baseline, participants with discordance had higher mean TG, lower TC, LDL, and HDL than non-discordant participants. The mean TC/HDL ratio in participants with discordance was (4.45±0.24) higher than that in non-discordant participants (4.07±0.48). At 1 year, only the mean HDL remained lower in discordant participants than in the non-discordant participants. The mean TC/HDL ratio in discordant participants (4.75±0.65) remained higher than that in non-discordant participants (4.16±0.64).
Table 3 shows the performance of the respective classifiers in predicting discordance. The random forest was the best-performing classifier and was selected for further study. Table 4 lists the baseline features used for the construction of the model predicting discordance at 1 year with their respective importance. The selected features were age, BMI, sFG score, TSH, and HOMA-IR; and free T, TG, HDL, LDL, TC, EE-15, EE-20, and EE-30. Among the baseline features, TG, HOMA-IR, BMI, and HDL had a high Gini index. All three drugs influenced discordance at 1 year with EE-30 pills having the maximal influence and explaining 6.0% of the variation.
Table 5 shows the adverse effects reported by the participants following drug use. Sixteen patients in the EE-15 arm reported breakthrough bleeding, which was significantly higher than that in the other two arms and required assurance and counseling of the participants by the investigators.
This study aimed to compare metabolic outcomes in women with lean PCOS who were administered different hormonal contraceptive pills. To the best of our knowledge, the present study is the first to evaluate the prevalence of TC/HDL-LDL discordance among these women. The baseline discordance was 26.0%, and participants with discordance had low HDL, higher TC/HDL ratio, and high TG compared to non-discordant participants. Following treatment with hormonal pills, the prevalence of discordance diminished to 18.0% at 1 year, but the mean HDL was low and TC/HDL remained higher among discordant participants than among non-discordant participants. A comparison between arms at 1 year showed a higher discordance in low-dose pill users (32.0%) than in ultralow-dose pill users (6.0% in EE-20 and 16.0% in EE-15). The mean TC and LDL levels were higher in the low-dose pill users than in the ultra-low-dose pill users. Lipoprotein indices at baseline (TG, LDL, HDL, and TC) explained almost a third of the variations in discordance at 1 year. All three pills influenced discordance at 1 year although there were differences in their respective Gini indices.
Studies in different populations have shown that discordance is a consistent marker for predicting ASCVD. The Framingham Offspring Cohort aged 40-75 years reported that individuals with apoB to non-HDL-C and LDL-C discordance had higher incidences of metabolic syndrome, hypertension, diabetes, and obesity [22]. The coronary artery risk development in young adults cohort from urban areas of the USA highlighted that almost 8.0% of young adults (aged 18-30 years) who had discordantly high apoB and low non-HDL-C had elevated atherogenic particle numbers in early adulthood [23]. A large population-based prospective study among middle-aged men and women from multiple districts of the USA reported that 26.0% of individuals had TC/HDL-C and LDL discordance and had a 24.0% greater risk of incident ASCVD than non-discordant individuals [24]. Discordance as a marker of future cardiac risk in reproductive-aged women with PCOS who are prone to dyslipidemia has not previously been evaluated. Studies from India reported that approximately 18.0% of young adolescent women from urban areas have PCOS [25]. All have reported dyslipidemia based on abnormalities in the individual lipoprotein index, and the prevalence varied between 27.0-33.0% [26].
This study primarily focused on the effects of the different pills on key metabolic parameters, including triglycerides, LDL, HDL, and TC. This deviates from a previous study by Romualdi et al. [27], who reported significant increases in triglyceride, LDL, HDL, and TC in users of both pill types after a year of treatment. Compared with EE-30, changes with EE-20 pills were milder, and the LDL/HDL ratio did not show a significant difference in either arm after treatment [27]. These discrepancies may stem from variables, such as ethnic diversity, baseline androgen levels, and disparities in the progestin components of the pills used in the two studies [27,28]. Population-based studies have shown that women with higher baseline androgen levels are more likely to show resistance to the effects of estrogen, and significant changes in lipoprotein levels following treatment may be less explicit [29,30]. In vitro studies have shown that exogenous estrogen at physiological concentrations activates type I estrogen receptor (ER) and elevates apoAI and apoCII levels, resulting in HDL synthesis. At supra-physiological serum concentrations, exogenous estrogen stimulates type II ER, which elevates apoE and apoB levels, especially where receptors are presensitized to estrogen, a scenario that is very likely in PCOS. The action of estrogen on type I receptors is inhibited by androgen [31]. The present study participants had normal baseline free T4 and at 1-year post-intervention, the free T4 was significantly reduced in all arms. Users of ultralow-dose pills showed an increase in HDL, while users of low-dose pills showed an increase in LDL and TC. This differential effect may result from variations in lipid oxidation induced by different steroidal hormones [32]. The concept of androgenic progestins potentially inducing anti-estrogenic effects that might influence lipid metabolism is also possible. Observations in one previous comparative study reported that EE 20 μg combined with Gestodene or Drosperinone increased HDL and apoA1 and EE 30 μg combined with Desogestrel or Levonorgestrel caused elevation of LDL in healthy premenopausal women [33].
Discordant participants had low HDL levels at baseline and after 1 year of treatment. HA is a known inducer of hepatic lipase (HL), which promotes the catabolism of HDL, and an inhibitor of the lecithin-cholesterol acetyltransferase enzyme, which results in the conversion of VLDL remnants to HDL. Enhanced HL activity promotes the formation of small dense LDL [34,35]. Discordant or non-discordant participants, however, did not show a difference in free T4 levels at baseline or after 1-year treatment with a hormonal pill. This might suggest an inherently higher sensitivity of discordant participants to free T4. This conjecture is supported by the fact that users of ultra-low-dose pills who had a higher free T4 after treatment had a decrease in the proportion of discordance at 1 year. In users of low-dose pills, despite lower free T4 discordance, was higher with almost a third of the discordant participants at baseline remaining discordant. However, we did not have a sufficient number of participants from each treatment arm to provide definitive opinions on this possibility.
Prescriptive models using linear classifiers have shown that age, sex, and three directly measured standard lipid parameters could explain 86.0% of the TC/HDL and apoB discordance in the adult USA population [21]. As most biological data do not follow a linear distribution, we selected random forest as a standard non-linear classifier for better accuracy, which adds to the novelty of this study. An additional observation of this study was that baseline HOMA-IR was an important predictor of discordance. In the present study, HOMA-IR reduced following treatment with ultra-low-dose pills, whereas low-dose pills elevated it at 1 year. In vitro studies have shown that unbound estrogen promotes IR by binding to insulin and insulin receptors and effects were dose dependent [36].
To date, definite evidence on the effects of various pill combinations on the risk of ASCVD in women with normal-weight PCOS is absent, and this study is the first to perform an randomized control trial between three pills of different compositions. The limitations include the fact that, being a prospective study of 1-year duration we could not observe definite cardiovascular outcomes which would require long-term follow-up of the cohort for a long duration. As the study was restricted to a particular ethnic group, the observed metabolic effects and inferences derived from the classifier may not be applicable to all populations [37]. In addition, the dietary habits and daily activities of a participant were not included as features in the model owing to potential inaccuracies in proper quantification following oral interviews of the participants. This reduces the performance and generalizability of the developed prediction model. The implications of this study emphasize the need for continued research on the in vivo metabolic consequences of steroidal hormone administration in women with PCOS, particularly at pharmacological doses. Overall, the present study establishes that pill composition influences lipid parameters at 1 year of regular use, and ultralow-dose pills might lower the risk of ASCVD in non-obese women with PCOS, as suggested by the decreased prevalence of TC/HDL discordance.
In conclusion, the intricate interplay between hormones and enzymes in women of reproductive age underscores the complex effects of hormonal pills on metabolic processes. The quest to identify safer, long-term combinations of these pills remains paramount. The intriguing finding that ultralow-dose pills can enhance the lipoprotein milieu, despite their limited efficacy in reducing androgen levels, raises important questions about their mechanisms of action. While a definitive explanation eludes the current understanding, it sheds light on the role of pill administration in metabolic responses in non-obese women with PCOS. However, the path toward a comprehensive mechanistic understanding requires further exploration.
Notes
Ethical approval
This study was approved by the institutional ethics committee of Chittaranjan Seva Sadan. The trial was prospectively registered in the Clinical Trial Registry of India (CTRI) with trial registration number CTRI/2018/02/011920 (registered on: 15/02/2018).
Fig. 1
Flow of study participants during study period. PCOS, polycystic ovarian syndrome; BMI, body mass index; COC, combined oral contraceptives; ITP, idiopathic thrombocytopenic purpura; EE, Ethinylestradiol.
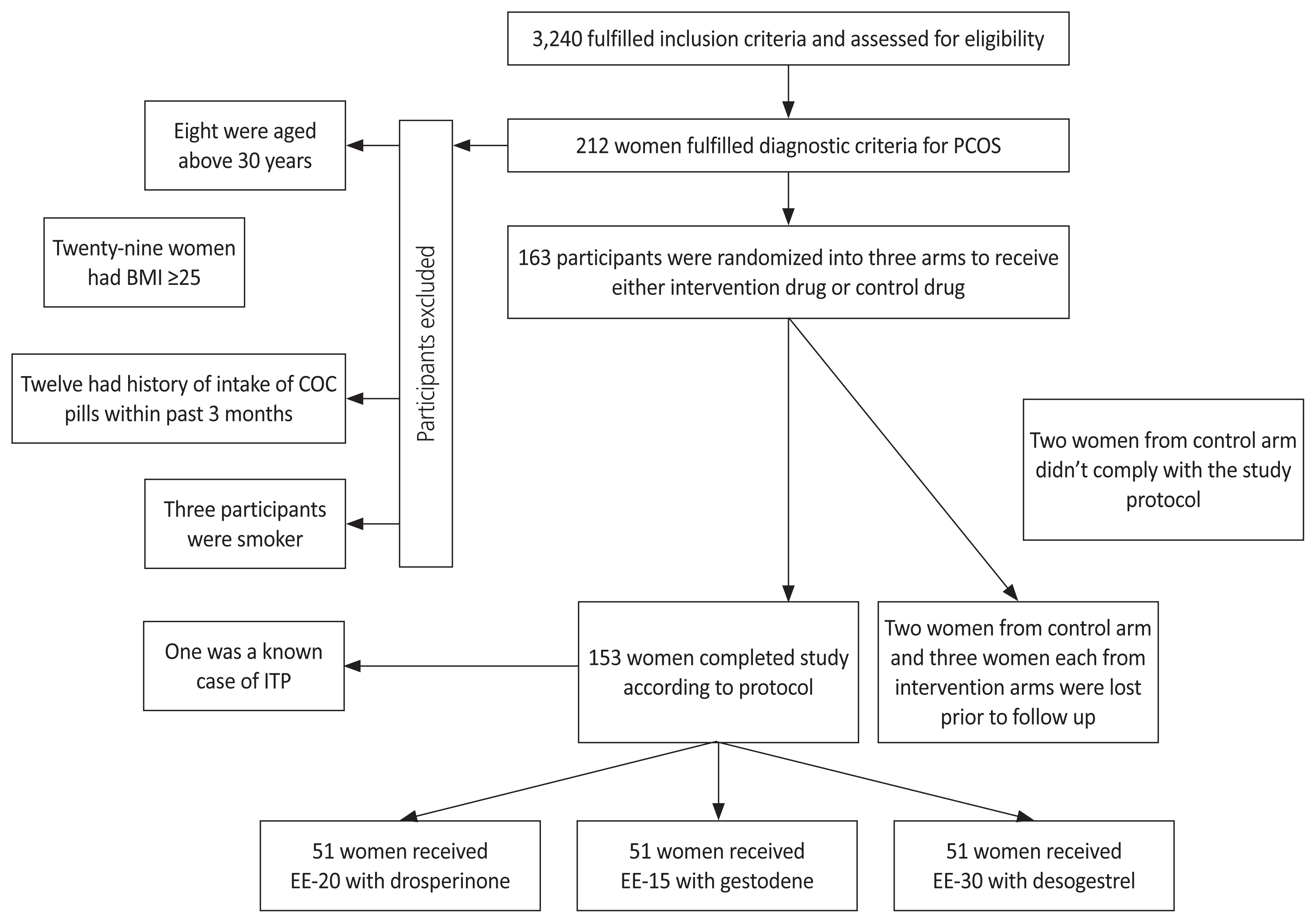
Table 1
Patient features at baseline and at 1 year for the three treatment arms
Analysis of variance (ANOVA) or Kruskal-Wallis test was used for comparisons between groups, followed by Tukey’s test if significant for continuous variables. The chi-square test was followed by Fischer’s exact test if significant for categorical variables.
Table 2
Differences in patient features between discordant and non-discordant group at baseline and after treatment
| Parameter | Discordant | Non-discordant |
|---|---|---|
| Number of participants at baseline | 42 | 111 |
| Number of participants after treatment | 27 | 126 |
| Age (yr) | 25.40±4.04 | 24.8±4.02 |
| BMI, baseline | 22.87±1.46 | 22.48±1.68 |
| BMI, after treatment | 24.08±2.0 | 24.21±2.48 |
| sFG, baseline | 2 | 2 |
| sFG, after treatment | 1 | 1 |
| TSH, baseline (μU/mL) | 3.48±1.63 | 3.60±1.57 |
| TSH, after treatment (μU/mL) | 2.86±1.28 | 2.54±1.16 |
| Free testosterone, baseline (ng/dL) | 1.76±1.04 | 1.64±1.11 |
| Free testosterone, after treatment (ng/dL) | 0.41±0.21 | 0.43±0.24 |
| HOMA-IR, baseline | 2.20±0.84 | 2.07±0.85 |
| HOMA-IR, after treatment | 2.08±0.77 | 2.12±0.94 |
| TG, baselinea) (mg/dL) | 137.21±38.41 | 116.94±33.15 |
| TG, after treatment (mg/dL) | 130.29±19.83 | 122.59±25.74 |
| TC, baselinea) (mg/dL) | 157.26±10.34 | 172.10±16.46 |
| TC, after treatment (mg/dL) | 178.44±19.67 | 175.09±21.53 |
| LDL, baselinea) (mg/dL) | 93.12±7.94 | 104.86±12.40 |
| LDL, after treatment (mg/dL) | 100.96±10.91 | 105.06±17.39 |
| HDL, baselinea) (mg/dL) | 35.42±3.05 | 42.65±5.16 |
| HDL, after treatmenta) (mg/dL) | 37.85±3.81 | 42.52±3.79 |
Table 3
Performance parameters of different regression models
Table 4
Importance of different participant features predicting discordance at 1 year
Table 5
Observed adverse effects with frequency in different groups
| Adverse effect | EE-20 | EE-15 | EE-30 |
|---|---|---|---|
| Nausea/bloating | 12 | 8 | 22 |
| Feeling of weight gain | 5 | 3 | 7 |
| Hair loss | 7 | 6 | 7 |
| Tiredness/sleepiness | 8 | 5 | 6 |
| Break through bleedinga) | 7 | 16 | 4 |
| Amenorrhea | 2 | 4 | 9 |
References
1. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018;110:364-79.




2. Roach RE, Helmerhorst FM, Lijfering WM, Stijnen T, Algra A, Dekkers OM. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev 2015;2015:CD011054.



3. de Bastos M, Stegeman BH, Rosendaal FR, Van Hylckama Vlieg A, Helmerhorst FM, Stijnen T, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev 2014;(3):CD010813.


4. Halperin IJ, Kumar SS, Stroup DF, Laredo SE. The association between the combined oral contraceptive pill and insulin resistance, dysglycemia and dyslipidemia in women with polycystic ovary syndrome: a systematic review and meta-analysis of observational studies. Hum Reprod 2011;26:191-201.


5. Amiri M, Ramezani Tehrani F, Nahidi F, Kabir A, Azizi F, Carmina E. Effects of oral contraceptives on metabolic profile in women with polycystic ovary syndrome: a meta-analysis comparing products containing cyproterone acetate with third generation progestins. Metabolism 2017;73:22-35.


6. Brill K, Then A, Beisiegel U, Jene A, Wünsch C, Leidenberger F. Investigation of the influence of two low-dose monophasic oral contraceptives containing 20 micrograms ethinylestradiol/75 micrograms gestodene and 30 micrograms ethinylestradiol/75 micrograms gestodene, on lipid metabolism in an open randomized trial. Contraception 1996;54:291-7.


7. Akerlund M, Almström E, Högstedt S, Nabrink M. Oral contraceptive tablets containing 20 and 30 micrograms of ethinyl estradiol with 150 micrograms desogestrel. Their influence on lipids, lipoproteins, sex hormone binding globulin and testosterone. Acta Obstet Gynecol Scand 1994;73:136-43.


8. Cibula D, Sindelka G, Hill M, Fanta M, Skrha J, Zivny J. Insulin sensitivity in non-obese women with polycystic ovary syndrome during treatment with oral contraceptives containing low-androgenic progestin. Hum Reprod 2002;17:76-82.


9. Dokras A, Playford M, Kris-Etherton PM, Kunselman AR, Stetter CM, Williams NI, et al. Impact of hormonal contraception and weight loss on high-density lipoprotein cholesterol efflux and lipoprotein particles in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2017;86:739-46.




10. Bhattacharya SM, Jha A, DasMukhopadhyay L. Comparison of two contraceptive pills containing drospirenone and 20 μg or 30 μg ethinyl estradiol for polycystic ovary syndrome. Int J Gynaecol Obstet 2016;132:210-3.



11. Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos GP. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab 2007;18:280-5.


12. Norman RJ, Mahabeer S, Masters S. Ethnic differences in insulin and glucose response to glucose between white and Indian women with polycystic ovary syndrome. Fertil Steril 1995;63:58-62.


13. Wijeyaratne CN, Balen AH, Barth JH, Belchetz PE. Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among South Asians and Caucasians: is there a difference? Clin Endocrinol (Oxf) 2002;57:343-50.



14. Cibula D, Hill M, Fanta M, Sindelka G, Zivny J. Does obesity diminish the positive effect of oral contraceptive treatment on hyperandrogenism in women with polycystic ovarian syndrome? Hum Reprod 2001;16:940-4.


15. Kim JJ, Chae SJ, Choi YM, Hwang KR, Song SH, Yoon SH, et al. Atherogenic changes in low-density lipoprotein particle profiles were not observed in non-obese women with polycystic ovary syndrome. Hum Reprod 2013;28:1354-60.


16. Chen MJ, Yang WS, Chen HF, Kuo JJ, Ho HN, Yang YS, et al. Increased follistatin levels after oral contraceptive treatment in obese and non-obese women with polycystic ovary syndrome. Hum Reprod 2010;25:779-85.


17. Cantey EP, Wilkins JT. Discordance between lipoprotein particle number and cholesterol content: an update. Curr Opin Endocrinol Diabetes Obes 2018;25:130-6.


18. Conway GS, Agrawal R, Betteridge DJ, Jacobs HS. Risk factors for coronary artery disease in lean and obese women with the polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992;37:119-25.


19. Zhu S, Zhang B, Jiang X, Li Z, Zhao S, Cui L, et al. Metabolic disturbances in non-obese women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril 2019;111:168-77.


20. Cook H, Brennan K, Azziz R. Reanalyzing the modified Ferriman-Gallwey score: is there a simpler method for assessing the extent of hirsutism? Fertil Steril 2011;96:1266-70.e1.



21. Elshazly MB, Quispe R, Michos ED, Sniderman AD, Toth PP, Banach M, et al. Patient-level discordance in population percentiles of the total cholesterol to high-density lipoprotein cholesterol ratio in comparison with low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol: the very large database of lipids study (VLDL-2B). Circulation 2015;132:667-76.



22. Pencina MJ, D’Agostino RB, Zdrojewski T, Williams K, Thanassoulis G, Furberg CD, et al. Apolipoprotein B improves risk assessment of future coronary heart disease in the Framingham Heart Study beyond LDL-C and non-HDL-C. Eur J Prev Cardiol 2015;22:1321-7.



23. Wilkins JT, Li RC, Sniderman A, Chan C, Lloyd-Jones DM. Discordance between apolipoprotein B and LDL-cholesterol in young adults predicts coronary artery calcification: the CARDIA study. J Am Coll Cardiol 2016;67:193-201.


24. Quispe R, Elshazly MB, Zhao D, Toth PP, Puri R, Virani SS, et al. Total cholesterol/HDL-cholesterol ratio discordance with LDL-cholesterol and non-HDL-cholesterol and incidence of atherosclerotic cardiovascular disease in primary prevention: the ARIC study. Eur J Prev Cardiol 2020;27:1597-605.



25. Sharma M, Khapre M, Saxena V, Kaushal P. Polycystic ovary syndrome among Indian adolescent girls - a systematic review and metanalysis. Nepal J Epidemiol 2021;11:1063-75.




26. Joshi SR, Anjana RM, Deepa M, Pradeepa R, Bhansali A, Dhandania VK, et al. Prevalence of dyslipidemia in urban and rural India: the ICMR-INDIAB study. PLoS One 2014;9:e96808.



27. Romualdi D, De Cicco S, Busacca M, Gagliano D, Lanzone A, Guido M. Clinical efficacy and metabolic impact of two different dosages of ethinyl-estradiol in association with drospirenone in normal-weight women with polycystic ovary syndrome: a randomized study. J Endocrinol Invest 2013;36:636-41.



28. de Medeiros SF. Risks, benefits size and clinical implications of combined oral contraceptive use in women with polycystic ovary syndrome. Reprod Biol Endocrinol 2017;15:93.


29. Lerchbaum E, Schwetz V, Rabe T, Giuliani A, Obermayer-Pietsch B. Hyperandrogenemia in polycystic ovary syndrome: exploration of the role of free testosterone and androstenedione in metabolic phenotype. PLoS One 2014;9:e108263.



30. Dumesic DA, Tulberg A, McNamara M, Grogan TR, Abbott DH, Naik R, et al. Serum testosterone to androstenedione ratio predicts metabolic health in normal-weight polycystic ovary syndrome women. J Endocr Soc 2021;5:bvab158.




31. Tam SP, Archer TK, Deeley RG. Biphasic effects of estrogen on apolipoprotein synthesis in human hepatoma cells: mechanism of antagonism by testosterone. Proc Natl Acad Sci U S A 1986;83:3111-5.



32. Zhu XD, Bonet B, Knopp RH. 17beta-estradiol, progesterone, and testosterone inversely modulate low-density lipoprotein oxidation and cytotoxicity in cultured placental trophoblast and macrophages. Am J Obstet Gynecol 1997;177:196-209.

33. Lüdicke F, Gaspard UJ, Demeyer F, Scheen A, Lefebvre P. Randomized controlled study of the influence of two low estrogen dose oral contraceptives containing gestodene or desogestrel on carbohydrate metabolism. Contraception 2002;66:411-5.


34. Legro RS, Dodson WC, Kris-Etherton PM, Kunselman AR, Stetter CM, Williams NI, et al. Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J Clin Endocrinol Metab 2015;100:4048-58.



- TOOLS




















