Fetal growth changes and prediction of selective fetal growth restriction following fetoscopic laser coagulation in twin-to-twin transfusion syndrome
Article information
Abstract
Objective
To investigate fetal growth changes and predictive factors for selective fetal growth restriction (sFGR) in patients with twin-to-twin transfusion syndrome (TTTS) after fetoscopic laser coagulation (FLC).
Methods
This retrospective study included twin-pregnant women with fetal TTTS who underwent FLC at our institution between 2011 and 2020. Twin pairs who survived at least 28 days after FLC and at least 28 days after birth were included. A paired t-test was used to compare the mean discordance between the estimated fetal weights at the FLC and the birth weights. The predictive factors for sFGR after FLC were evaluated using univariate and multivariate logistic regression analyses.
Results
A total of 119 eligible pairs of patients who underwent FLC were analyzed. The weight percentile at birth significantly decreased after FLC in the recipients (53.7±30.4 percentile vs. 43.7±28.0 percentile; P<0.001), but increased in the donors (11.5±17.1 percentile vs. 20.7±22.8 percentile; P<0.001). Additionally, the mean weight discordance of twin pairs significantly decreased after FLC (23.9%±12.7% vs. 17.3%±15.7%; P<0.001). After FLC, Quintero stage ≥3, pre-FLC sFGR, abnormal cord insertion, and post-FLC abnormal umbilical artery Doppler (UAD) were all significantly higher in the sFGR group than the non-sFGR group. The prediction model using these variables indicated that the area under the receiver operating characteristic curve was 0.898.
Conclusion
The recipient weight percentile decreased, whereas donor growth increased, resulting in reduced weight discordance after FLC. The Quintero stage, pre-FLC sFGR, and post-FLC abnormal UAD were useful predictors of sFGR after FLC in TTTS.
Introduction
Twin-to-twin transfusion syndrome (TTTS) is a unique complication caused by vascular anastomosis in monochorionic twins, which leads to volume and nutritional depletion in the donor fetus and volume overload and heart failure in the recipient fetus [1,2]. Fetoscopic laser coagulation (FLC) of communicating vessels is the treatment of choice for TTTS [3,4]. TTTS is commonly accompanied by impaired fetal growth [5,6], which may be attributed to abnormal placental sharing and unbalanced blood flow through vascular communications within a common placenta [7,8].
Selective fetal growth restriction (sFGR) is a complication observed in 10–15% of monochorionic twin pregnancies [9]. sFGR increases the risk of perinatal mortality and morbidity [10–12], and fetal growth restriction is often caused by various etiologies [13]. Several studies have reported fetal growth after FLC in twins with TTTS. However, the results were inconsistent [1,7,14,15]. These studies reported that weight discordance between fetuses decreased; however, the cause of the decrease in fetal weight, whether the recipients’ growth slowed and/or the donors’ growth accelerated, was reported differently in each study. In addition, these studies focused on the effects of obliteration of the vascular anastomosis between the recipient and donor twins on the growth of both fetuses. However, a multifaceted approach is required to comprehensively understand the risks associated with sFGR. Moreover, no recent data have been reported on this topic.
Considering that the FLC technique has a steep learning curve and that the perinatal outcomes of FLC have recently improved [16,17], it is clinically relevant to investigate potential factors for predicting fetal growth after FLC. This study aimed to investigate fetal growth changes and analyze the parameters that predict sFGR following FLC in TTTS.
Materials and methods
1. Study design
This retrospective cohort study included women with twin pregnancies diagnosed with TTTS who underwent FLC at our institution between 2011 and 2020. To ensure that the change in fetal growth after FLC could be sufficiently evaluated, the inclusion criteria were as follows: 1) fetuses delivered at least 28 days after FLC and 2) neonates surviving longer than 28 days after birth. Subjects with triplet pregnancies, fetuses with major anomalies, post-FLC twin anemia polycythemia sequence, or loss of follow-up were excluded. Patients requiring additional FLC or treatment were also excluded. Antenatal and neonatal information was acquired from our prospectively collected institutional database. The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB number: 2022-0536).
The weight percentiles and discordance of the weights of the twin pairs were compared before and after the FLC. According to the presence or absence of sFGR after FLC, 1) the clinical findings: Quintero stage at FLC, pre-FLC sFGR, gestational age at diagnosis of TTTS and FLC, interval to delivery from FLC, and location of cord insertion and 2) the sonographic findings after FLC: visible bladder, deepest vertical pocket (DVP) of amniotic fluid ≥2 cm, and umbilical artery Doppler were compared as the predictive factors. Subsequently, a prediction model using these variables indicated that the area under the receiver operating characteristic curve was developed, and a nomogram was plotted to predict sFGR after FLC.
2. Diagnosis of TTTS and perioperative Doppler assessment
TTTS was diagnosed based on the sonographic findings of 1) a maximum vertical pocket >8 cm in one sac (polyhydramnios) and 2) a maximum vertical pocket <2 cm in the other sac (oligohydramnios) at any gestational age [18,19]. All subjects underwent ultrasonography within 24 hours of FLC, and the estimated fetal weight (EFW) and DVP of the amniotic fluid were measured. Once TTTS was diagnosed, pulsed-wave Doppler waveforms were obtained from the floating portion of the umbilical artery within an insonation angle of 15°. Abnormal umbilical artery Doppler (UAD) was defined based on UAD waveforms as the absence of end-diastolic flow, or intermittent or persistent reversed end-diastolic flow within 7 days after FLC. Subsequently, ultrasonography was serially performed for follow-up. All ultrasonographic measurements were performed using an A30, WS80A, HERA W10 (Samsung Medison Co., Ltd., Seoul, Korea), Volusion E8, or E10 Expert (General Electric Healthcare Austria GmbH & Co. OG, Pfaffing, Austria) ultrasound devices with a 2–6-MHz transabdominal probe.
3. Fetoscopic laser coagulation
Based on the Quintero stage, TTTS with stage I or higher, presenting symptomatic polyhydramnios or cardiac dysfunction in the recipients underwent FLC [19]. Between 2011 and 2014, the selective coagulation technique was used. Subsequently, the Solomon technique has been used at our institution since 2014 for the coagulation of vessel anastomoses between recipients and donors, as previously described in detail [17]. FLC was performed by the same fetal therapy team, consisting of two maternal-fetal medicine specialists, three experienced nurses, and expert-trained assistants specializing in maternal-fetal medicine.
4. Definition of selective fetal growth restriction
The sFGR was defined as EFW (or birth weight) in one of the fetuses less than the 10th percentile for gestational age and greater than 25.0% inter-twin EFW (or birth weight) discordance [20]. The weight percentile was determined according to the World Health Organization fetal growth charts (https://srhr.org/fetalgrowthcalculator/#/) [21].
5. Diagnosis of abnormal cord insertion
Abnormal cord insertion was defined as marginal and velamentous cord insertion [22]. The criteria for marginal cord insertion were based on <2 cm attachment of the umbilical cord to the margin of the placenta, whereas that of velamentous cord insertion was attachment to the membrane outside the placental disc.
6. Statistical analysis
IBM SPSS Statistics for Windows version 21 (IBM Corp., Armonk, NY, USA) and R statistical software (version 3.4.4; R Foundation for Statistical Computing, Vienna, Austria) were used to analyze all data. Proportions were compared using the Pearson’s chi-square test, Fisher’s exact test, or McNemar’s test. Continuous variables were compared using independent and paired t-tests where appropriate. Univariate logistic regression analysis was employed to identify predictors for sFGR from the four variables. Variables with P-values <0.05 were subsequently incorporated into a multivariate logistic regression model using the enter method. To evaluate the predictive model’s performance, we constructed a receiver operating characteristic (ROC) curve and calculated the area under the ROC curve (AUC) using the “pROC” R package [23]. Finally, a nomogram consisting of clinically meaningful predictive factors was constructed with the “rms” R package [24]. Statistical significance was set at a two-sided P-value <0.05 [25].
Results
1. Clinical characteristics of study population
A flowchart of the patient selection process is shown in Fig. 1. Between 2011 and 2020, 229 twin pairs were diagnosed with TTTS and underwent FLC. One hundred and three subjects were excluded due to triplet pregnancy (n=4), fetuses with major anomalies (n=1), post-FLC twin anemia polycythemia sequence (n=2), death (n=89), and lost to follow-up (n=7). Among the 126 pairs of twins that survived at least 28 days after birth, 119 pairs of twins delivered at least 28 days after FLC were included.
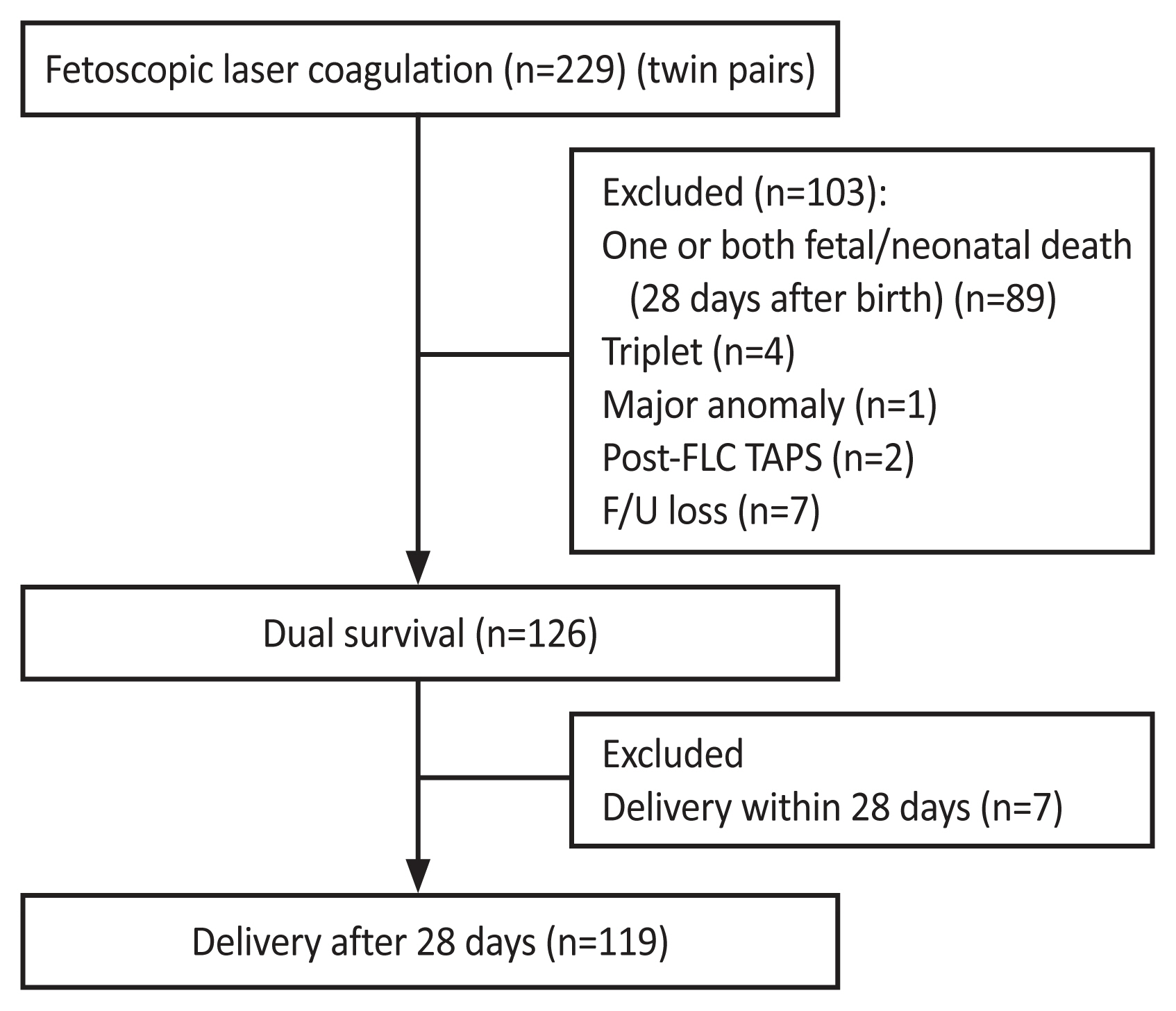
Flow chart of the study group. FLC, fetoscopic laser coagulation; TAPS, twin anemia polycythemia sequence; F/U, follow-up.
The mean gestational ages at diagnosis, FLC, and delivery were 20.2±2.5, 20.6±2.5, and 32.9±3.2, respectively (Table 1). The mean interval between FLC and delivery was 86.3±28.2 days. According to Quintero stage at FLC, there were 21 stage I (17.6%), 21 stage II (17.6%), 72 stage III (60.5%), and five stage IV (4.2%) patients.
2. Inter-twin discordance and a proportion of the growth restriction between FLC
The weight percentile at birth significantly decreased after FLC in the recipients (53.7±30.4 percentile vs. 43.7±28.0 percentile; P<0.001), but significantly increased in the donors (11.5±17.1 percentile vs. 20.7±22.8 percentile; P<0.001) (Table 2). Additionally, the mean weight discordance of twin pairs significantly decreased after FLC (23.9±12.7% vs. 17.3±15.7%; P<0.001). The proportion of small for gestational age also significantly decreased after FLC in the donors (74.4% vs. 56.3%; P<0.01) but not in the recipients (12.6% vs. 14.3%; P=NS). The number of sFGR decreased from 55 to 29 twins after FLC, indicating a 47.0% improvement in sFGR.
3. Clinical and sonographic features of the sFGR group after FLC
Of the 119 surviving twin pairs, 29 had sFGR at birth. Quintero stage ≥3, sFGR at FLC, abnormal cord insertion, and post-FLC abnormal UAD were all significantly higher in the sFGR group than in the non-sFGR group (Table 3). However, all other clinical features, including visible bladder, DVP of amniotic fluid ≥2 cm, gestational age at diagnosis, FLC, delivery, and interval to delivery, were not significantly different between the two groups.
4. Prediction for sFGR after FLC
In logistic regression analyses, Quintero stage ≥3, pre-FLC sFGR, and post-FLC abnormal UAD remained significant independent factors in multivariate analysis (Table 4). Among them, post-FLC abnormal UAD showed the highest effect on sFGR (odd ratio [OR], 24.1; 95% confidence interval [CI], 5.1–113.2; P<0.001], followed by pre-FLC sFGR (OR, 11.0; 95% CI, 2.7–44.6; P=0.001) and Quintero stage ≥3 (OR, 1.6; 95% CI, 0.5–6.4; P<0.01). Using these independent variables in the multivariate predictive model, the AUC was 0.898 (Fig. 2).

Logistic regression analyses to predict selective fetal growth restriction following fetoscopic laser coagulation
Discussion
In this study, the weight discordance of twin pairs significantly decreased due to the slowdown in growth of the recipients and catch-up growth of the donors. Approximately half of the TTTS with sFGR before FLC demonstrated improvement (26/55; 47.0%). Furthermore, the Quintero stage, pre-FLC sFGR, and abnormal UAD after FLC were useful predictive factors for sFGR, with an AUC of 0.898. These findings suggest that obliteration of the inter-twin vascular connection by the FLC in a shared placenta could affect the growth of TTTS fetuses. A nomogram for predicting sFGR after FLC was developed using these factors.
Several papers have been published regarding fetal growth after FLC [1,7,14,15]; among them, two studies on fetal growth after FLC reported that the mean discordances were 26.6–28.0% before FLC and 18.0–18.4% at birth, respectively [7,14], which is consistent with our findings. Both studies showed that inter-twin weight discordance decreased after FLC. However, fetal growth has been inconsistent among relevant studies. In studies by Moreira et al. [14] and Maschke et al. [15], the growth rate decreased in the recipient, but was maintained in the donor. However, Chmait et al. [1] reported that donor growth was accelerated, whereas that of the recipient remained unchanged after FLC. In the current study, there was an opposite relationship between the growth of recipients and donors, which eventually led to a significant reduction in the weight discordance of the twin pairs (Table 5). The discrepancy between these results might be due to differences in the ablation locations of the vascular anastomosis. At our institution, to save the donor twin’s area, FLC focuses on ablating the vascular anastomosis in the recipient’s territory as much as possible. A properly chosen ablation site could further salvage the donor area, which might lead to accelerated catch-up growth in the donor fetus.
Interestingly, there was no significant difference in visible bladder and DVP of amniotic fluid ≥2 of the donor twins between the sFGR and non-FGR groups. We assumed that FLC might have successfully treated both groups, as nearly all fetuses demonstrated improvement in both findings. This finding suggests that these two parameters reflect the success of the FLC.
UAD reflects fetal conditions and has been shown to be beneficial in clinical conditions characterized by placental insufficiency and chronic nutritive and hypoxic stress in the fetus [13]. An abnormal UAD indicates reduced pressure and perfusion in the smaller twin, which can cause diminished placental and fetal growth [26]. Since the Quintero stage and abnormal cord insertion are static factors in terms of pathophysiology, the state of UAD might be a clinically manageable factor for fetal growth after FLC in TTTS.
The strength of this study is the long-term collection of data from a single center with the same team members consisting of operators, assistants, and nurses, which minimized operator-dependent variability. In addition, unlike the “selective method” implemented in previous studies [1,7], our study mostly applied the “Solomon technique” when FLC was performed, which resulted in a functionally dichorionic placenta and subsequently lowered the influence of vascular anastomosis on fetal growth. Therefore, it may aid in counseling patients with TTTS regarding future growth after FLC.
There are limitations to this study, other than its retrospective design. Considering that this study was conducted at a single center in South Korea, our results may not be reproducible in other centers with different cohorts.
In conclusion, the weight discordance among the twin pairs significantly decreased after FLC. The Quintero stage, pre-FLC sFGR, and post-FLC abnormal UAD proved to be useful predictors for sFGR after FLC in TTTS. Consideration of these factors would help clinicians predict catch-up growth in the donor fetus and enable appropriate clinical counseling and management for patients with TTTS after FLC.
Notes
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB number: 2022-0536).
Patient consent
Informed consent was not required because of the retrospective nature of the study.
Funding information
None.




