High prevalence of hypovitaminosis D3 among pregnant women in central Iran: correlation with newborn vitamin D3 levels and negative association with gestational age
Article information
Abstract
Objective
Hypovitaminosis D3 is a significant concern among pregnant women and their newborns because vitamin D3 (Vit-D3) plays a crucial role in embryonic growth, development, and health. This study aimed to evaluate the Vit-D3 status of a group of pregnant Iranian women and its association with newborn Vit-D3 levels, medical and clinical indices after delivery.
Methods
A total of 206 pregnant women and their newborns were assessed for Vit-D3 levels and their correlation with gestational age. Mean±standard deviation (SD) or the orders (non-parametric tests) of variables were compared, and correlation estimations were performed to elucidate any differences or associations between groups, with a confidence interval of at least 0.95.
Results
The mean±SD of mothers’ age and gestational age were 29.65±6.18 years and 35.59±1.6 weeks, respectively. Neonatal Vit-D3 levels were associated with maternal age. Using a 30 ng/mL cutoff point for serum Vit-D3 levels, 83.5% of pregnant women and 84.7% of newborns had hypovitaminosis D3. The average Vit-D3 levels of mothers and newborns at delivery time were 23.5±8.07 ng/mL and 20.76±9.14 ng/mL, respectively. Newborn Vit-D3 levels were positively correlated with maternal Vit-D3 serum levels (R=0.744; P<0.001) and gestational age (R=0.161; P=0.022). In newborns, head circumference was inversely correlated with bilirubin level (R=−0.302; P<0.001) but directly associated with weight (R=0.640; P<0.001).
Conclusion
Hypovitaminosis D3 remains a significant challenge for pregnant Iranian women. Maternal Vit-D3 levels provide for the newborn’s needs, particularly in the late stages of pregnancy. Therefore, Vit-D3 supplementation and regular monitoring are essential for pregnant women and their newborns.
Introduction
Hypovitaminosis D3 has become an increasingly prevalent problem worldwide [1]. Pregnant women, neonates, and breastfed infants are highly susceptible to vitamin D3 (Vit-D3) deficiency because of its essential role as a micronutrient [2]. The mother is the sole source of Vit-D3 for the fetus, and her nutritional status has a significant impact on embryonic growth, development, and overall health [3]. Vit-D3 supplementation and reception from the mother’s circulation and follicular fluid are crucial for maintaining embryonic characteristics, normal fertilization, implantation rate, placental development, and all stages of pregnancy [4–6].
Hypovitaminosis D3 is prevalent among pregnant women in Iran [7–9]. Studies have reported that maternal hypovitaminosis D3 may be associated with newborn problems such as skeletal, cardiovascular, respiratory, and nervous system involvement. Low birth weight, reduced calcium levels, retarded growth during and after birth, bone fractures, and autoimmune diseases are associated with low Vit-D3 levels [10]. Other outcomes of hypovitaminosis D3 include miscarriage, preeclampsia, gestational diabetes, maternal infection, and preterm delivery. Moreover, neonatal hyperbilirubinemia, early sepsis, low quality of health, and the need for cesarean delivery are associated with low maternal Vit-D3 levels [11–13].
Despite the frequent evidence regarding the outcomes of hypovitaminosis D3 in pregnant mothers and newborns, other studies have shown inconsistent results with these findings [14–16]. In this study, we report a significant prevalence of hypovitaminosis D3 in mothers and neonates. Also, interesting results of neonate's dependence to mother's Vit-D3 were obtained. Furthermore, contrary to previous reports, the anatomical, health, and clinical status of the fetus may be independent of the mother’s Vit-D3 levels.
Materials and methods
1. Method of investigation
This prospective, cross-sectional study evaluated 210 pregnant women referred to an educational hospital for pregnancy termination. This study aimed to assess the Vit-D3 levels in maternal and fetal samples. Blood specimens were collected from pregnant mothers and umbilical cords after delivery to determine hypovitaminosis D3, a condition characterized by low levels of Vit-D3 in the body [17,18]. Based on the exclusion criteria, four mothers were excluded from the study, leaving 206 mothers and neonates for inclusion.
2. Setting and ethical issues
This study was conducted in the maternity ward of a university hospital in Arak City, Markazi Province, Iran. We informed the enrolled women of the study’s purpose and obtained their written informed consent. Each patient was informed that the study would have no adverse effects on their pregnancy or newborn. The study incurred no cost to the patients or their families, and they were free to withdraw from study inclusion at any time. Patient anonymity was maintained, and private data were managed by a single investigator.
The exclusion criteria included a history of or recent involvement with chronic hypertension, diabetes, cardiovascular diseases, rheumatologic disorders such as systemic lupus erythematosus and rheumatoid arthritis, and hepatic and kidney problems. Patients receiving therapeutic regimens affecting Vit-D3 metabolism or absorption (corticosteroids, orlistat, phenytoin, and phenobarbital) were also excluded. Finally, we tested 206 mothers and umbilical cord blood (UCB) samples for Vit-D3 levels.
Furthermore, the intrauterine growth restriction (IUGR) term describes a condition in which a fetus fails to grow at a normal rate in the uterus; it is typically diagnosed when a fetus’s estimated weight is below the 10th percentile of its gestational age [19].
Apgar score is a standardized assessment tool used to evaluate the physical condition of newborns immediately after birth. The Apgar score was calculated based on five criteria as follows: heart rate, respiratory effort, muscle tone, reflex irritability, and color. The Apgar score is typically evaluated 1 minute and 5 minutes after birth [20]. The first-minute Apgar score provides an initial assessment of the newborn’s physical condition immediately after birth, while the 5-minute Apgar score provides a more comprehensive evaluation of the newborn’s response to resuscitative measures and overall condition. The Apgar score was calculated on a scale of 0–10, with a score of 10 indicating the highest level of physical health, and 0 indicating the lowest level. A score of 7 is generally considered normal, whereas a score of 3 is considered critically low and may require immediate resuscitation [21].
Blood samples from mothers and umbilical cords were collected during natural or cesarean delivery by midwives and nurses. A demographic data list was completed for each patient along with their Vit-D3 serum levels. Additionally, we recorded UCB bilirubin concentration, Vit-D3 levels, neonate weight, height, head circumference, and gestational age (in weeks). We also noted instances of small for gestational age (SGA), IUGR, Apgar scores at 1 minute and 5 minutes after birth, intraventricular hemorrhage (IVH), neonatal death, early sepsis, and hyperbilirubinemia requiring hospitalization. SGA refers to newborns whose birth weight is below the 10th percentile for their gestational age, whereas IUGR is a condition in which a fetus fails to grow at a normal rate in the uterus and is typically diagnosed when a fetus’s estimated weight is below the 10th percentile for gestational age [19].
Apgar score is a standardized assessment tool used to evaluate the physical condition of newborns immediately after birth. The score is based on the following five criteria: heart rate, respiratory effort, muscle tone, reflex irritability, and color. The Apgar score was evaluated 1 minute and 5 minutes after birth, providing an initial assessment of the newborn’s physical condition and a more comprehensive evaluation of their response to resuscitative measures and overall condition, respectively. The score was calculated on a scale of 0–10, with a score of 10 indicating the highest level of physical health and a score of 0 indicating the lowest level. A score of 7 is generally considered normal, whereas a score of 3 is considered critically low and may require immediate resuscitation [21].
One month after birth, we contacted the parents for follow-up regarding any occurrence of neonatal jaundice that required emergency hospitalization. Incomplete checklists were reviewed by examining patient records or telephone calls. We also surveyed for any instances of IVH and sepsis occurring 1 month after delivery.
To measure Vit-D3 levels, we used an approved clinical laboratory method based on enzyme-linked immunosorbent assay (ELISA) (PishtazTeb Zaman Co., Tehran, Iran). According to the endocrine society’s clinical practice guidelines on vitamin D deficiency, the normal range for serum 25-hydroxyvitamin D3, the major circulating form of Vit-D3, according to the endocrine society’s clinical practice guideline on vitamin D deficiency, is 30–100 ng/mL (75–250 nmol/L) [22]. Therefore, we used a cutoff point of 30 ng/mL (lower limit) to describe hypovitaminosis D3 in our study. Participants were categorized into three groups based on the endocrine society’s clinical practice guideline on vitamin D deficiency, which provides the following definitions for Vit-D3 status based on serum levels: 1) sufficient group with serum Vit-D3 levels of 30–100 ng/mL; 2) insufficient group with serum Vit-D3 levels of 20–29 ng/mL; 3) deficient group with serum Vit-D3 levels below 20 ng/mL.
UCB bilirubin levels were measured using a biochemical procedure (Pars Azmon Co., Karaj, Iran) and an automatic chemistry analyzer in the hospital’s clinical laboratory. We used the seca digital metering system (seca 336, Hamburg, Germany) to determine the neonates’ height and weight (sensitivity: 1 cm and 1 g; respectively). We determined the Apgar scores at 1 and 5 minutes after birth using a standard Apgar table. The Apgar score includes five items: appearance (skin color), pulse, grimace (reflex irritability), activity (muscle tone), and respiration. Each item was assigned a score of 0, 1, or 2, with a maximum Apgar score of 10 and a minimum score of zero.
3. Data analysis
Statistical data analysis was performed using the Stata Statistical software ver. 11 (StataCorp LP, College Station, TX, USA). Depending on the type of variable, we conducted mean±standard deviation (SD) comparisons using independent samples t-tests or non-parametric tests, such as Kruskal-Wallis and Mann-Whitney tests, as well as a one-way analysis of variance (ANOVA). We employed the chi-square test to evaluate the distribution of nominal variables between the groups, and Pearson’s correlation was used to determine the association between variables. Statistical significance was at P<0.05.
Results
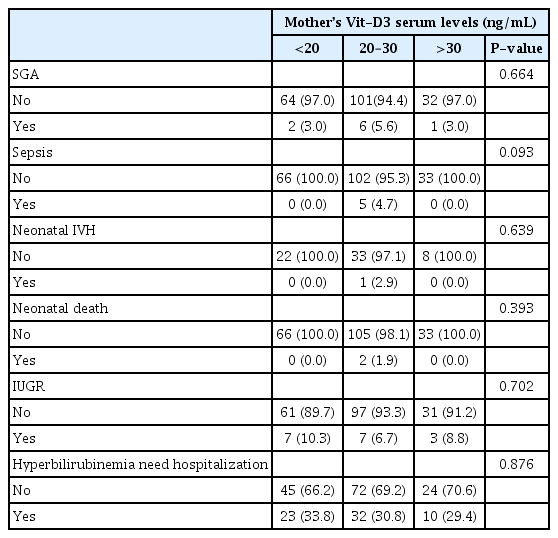
The mean±SD of mothers’ age and gestational ages were 29.65±6.18 (range, 17–45 years) and 35.59±1.6 weeks (range, 32–47 weeks), respectively. Neonatal Vit-D3 levels positively correlated with maternal age (R=0.191; P=0.006). No significant difference in the serum levels of Vit-D3 was observed among mothers with different educational levels (P=0.111). The Vit-D3 levels of mothers (P=0.612; Fig. 1A) and newborns (P=0.377; Fig. 1B) from rural areas were not significantly different from those from urban areas. Table 1 displays the frequency (%) of various neonatal disorders in the newborns studied, based on Vit-D3 levels. Table 2 presents the correlation coefficients between the variables examined, and Table 3 provides the selected demographic and descriptive data for the mothers and neonates who participated in the current study.
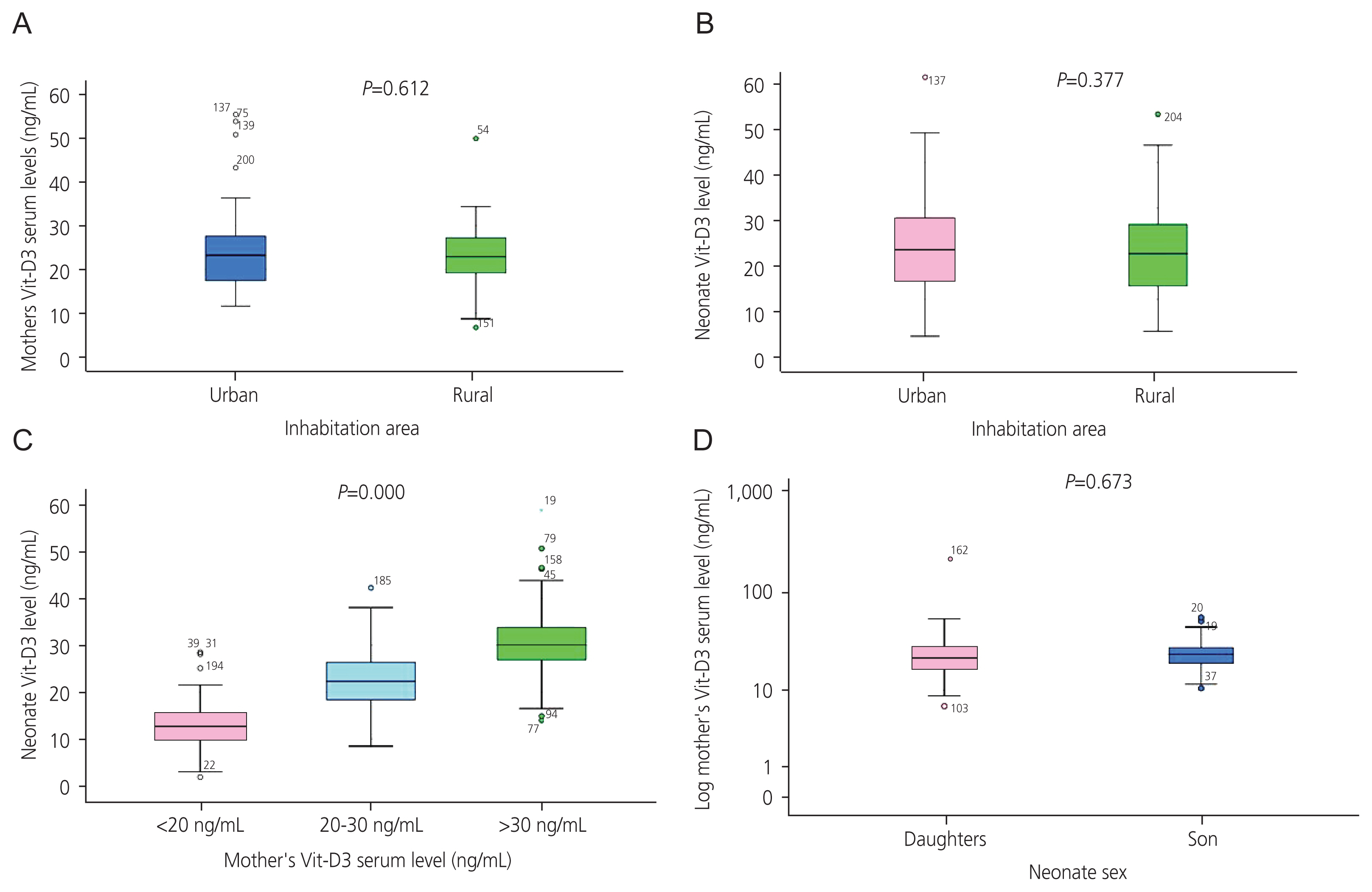
Comparison of mean±standard of maternal (A) and neonatal (B) Vit-D3 levels between urban and rural inhabitants. Inhabitation area did not impact the levels of maternal or neonatal Vit-D3. Mothers with higher levels of Vit-D3 gave birth to neonates with higher mean values of Vit-D3 (C), which is a significant finding. The mean values of maternal Vit-D3 levels did not differ significantly between mothers bearing sons or daughters (D). Vit-D3, vitamin D3.
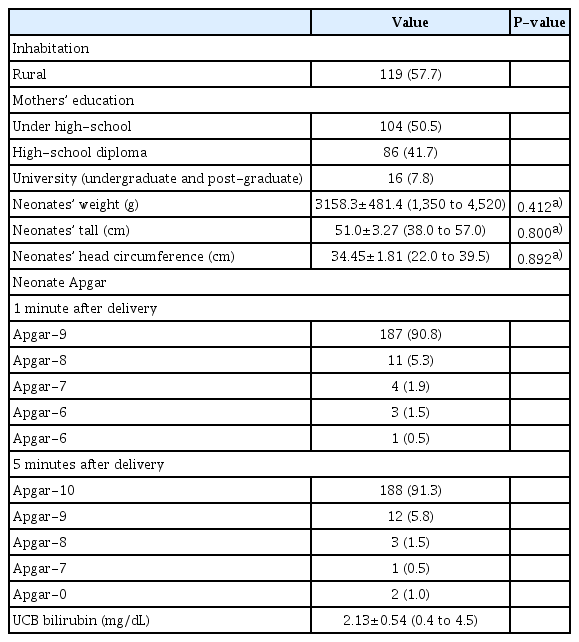
Selected demographic and descriptive data of mothers and neonates who participated in the current study
As stated in the materials and methods section, we considered the normal range of serum Vit-D3 levels as 30–100 ng/mL, based on the endocrine society’s clinical practice guideline on vitamin D deficiency [22]. Using a cutoff point of 30 ng/mL, we identified 172 (83.5%) individuals with hypovitaminosis D3. However, the mean±SD of Vit-D3 serum levels for mothers at delivery time was 23.5±8.07 ng/mL (min, 6.7 ng/mL; max, 55.4 ng/mL). Only 34 mothers (16.5%) had sufficient (>30 ng/mL), 104 (50.5%) had insufficient (20–30 ng/mL), and 68 (33%) had deficient (<20 ng/mL) Vit-D3 values.
Mothers’ Vit-D3 levels (mean±SD, 23.5±8.07 ng/mL) were strongly correlated with their neonates’ Vit-D3 levels (mean±SD, 20.76±9.14) (R=0.744; P=0.000) (Table 2). This indicates that mothers with higher Vit-D3 levels give birth to neonates with higher UCB Vit-D3. Fig. 1C compares the three groups of mothers: <20, those with 20–30 ng/mL, and >30 ng/mL Vit-D3 serum levels. Statistically, mothers in the >30 ng/mL group had neonates with the highest Vit-D3 levels, followed by mothers in the 20–30 ng/mL group; the lowest were in the <20 ng/mL group (P=0.000). This finding suggests that the fetus depends on the mother’s Vit-D3 sources during embryonic life. Again, using the 30 ng/mL cutoff point for serum Vit-D3 levels in neonates, 171 (84.7%) of the UCB samples indicated hypovitaminosis D3 among newborns.
In total, 88 (42.7%) male neonates (Vit-D3 mean±SD, 23.92±7.9) and 118 (57.3%) female neonates (Vit-D3 mean±SD, 23.24±8.4) demonstrated no significant difference in Vit-D3 serum levels (P=0.661). Their mothers also demonstrated no significant difference in Vit-D3 levels (P=0.673; Fig. 1D). The UCB Vit-D3 mean±SD was 20.82±9.15 ng/mL (min, 1.9 ng/mL; max, 58.7 ng/mL) at the time of delivery. Only 31 newborns (15.3%) had sufficient (>30 ng/mL), 70 (34.7%) had insufficient (20–30 ng/mL), and 101 (50%) had deficient (<20 ng/mL) Vit-D3 serum levels. Neonatal Vit-D3 positively correlated with gestational age (R=0.161; P=0.022), indicating that older fetuses had higher levels of Vit-D3.
Fig. 2 displays the non-significant mean±SD of newborns’ weight (Fig. 2A) (P=0.412), height (Fig. 2B) (P=0.800), and head circumference (Fig. 2C) (P=0.892) among mothers categorized into three groups based on Vit-D3 levels. This indicates that the mothers’ Vit-D3 levels did not affect the physical growth of the newborns in our study. Head circumference negatively correlated with UCB bilirubin levels (R=−0.302; P=0.000) and positively correlated with neonatal weight (R=0.640; P=0.000). The mother’s Vit-D3 level did not have a significant impact on UCB bilirubin concentration (P=0.696; Fig. 2D). However, in the follow-up survey, 63 newborns (34.4%) required phototherapy on subsequent days after hospital discharge. Of these, 56/63 (88.9%) received phototherapy at the hospital and 7/63 (11.1%) at home.

Comparison of mean±standard of newborn weight (A) and height (B) among mothers with Vit-D3 levels <20 ng/mL, 20–30 ng/mL, and >30 ng/mL. Insignificant results suggest that maternal Vit-D3 levels are not a significant factor in newborn weight or height. Inhabitation area did not affect the levels of maternal or neonatal Vit-D3. Mothers with higher levels of Vit-D3 gave birth to neonates with higher mean values of Vit-D3 (C), which is a significant finding. The mean values of maternal Vit-D3 levels did not differ significantly between mothers bearing sons or daughters (D). Vit-D3, vitamin D3.
The Vit-D3 levels in the mothers and UCB samples did not differ significantly between cesarean and natural deliveries (P=0.374) (Fig. 3). No significant difference in Apgar values was observed between the Vit-D3 categories (Vit-D3 <20 or 20–30 and >30 ng/mL) of newborns (P=0.491 for the 1 minute Apgar and P=0.534 for the 5-minute Apgar) or mothers (P=0.221 for the 1 minute Apgar and P=0.69 for the 5-minute Apgar) (Table 3).

Comparison of mean±standard of neonatal Vit-D3 between newborns delivered naturally or by cesarean section. The mean value of Vit-D3 in umbilical cord blood samples did not differ significantly between newborns delivered naturally or by cesarean section. Vit-D3, vitamin D3.
We categorized the education levels of the husbands into under high-school, high-school, and university levels and identified no significant differences between these three groups when comparing Vit-D3 levels in mothers (ANOVA P=0.43) or newborns (ANOVA P=0.345) (detailed data not presented).
Neonatal death (n=2; P=0.393), first-degree IVH (n=1; P=0.639), sepsis occurrence (n=5; P=0.093), growth retardation indices SGA (n=9; P=0.664), and IUGR (n=17; P=0.702) were independent of the mother’s Vit-D3 (Table 1). The distribution of newborns requiring hospitalization for hyperbilirubinemia treatment on subsequent days did not differ significantly when we compared mothers in the three categories of Vit-D3 serum levels (P=0.876; Table 1).
Mothers’ age and newborn Vit-D3 scores were positively and significantly correlated (R=0.191; P=0.006). Additionally, the mother’s age was positively and strongly correlated with body mass index (BMI) (R=0.219; P=0.003), but negatively and significantly correlated with gestational age (R=−0.144; P=0.041). However, maternal age did not significantly correlate with mother’s Vit-D3 serum levels (R=0.062; P=0.205).
The association between mothers’ Vit-D3 and newborn UCB Vit-D3 was highly significant (R=0.744; P=0.000), indicating a direct dependence of the newborn’s Vit-D3 on the mother’s Vit-D3 sources. Neonates with lower weights had higher UCB bilirubin levels (R=−0.363; P=0.000) and smaller head circumferences (R=−0.302; P=0.000). Neonates with longer gestational ages had higher weights (R=0.304; P=0.000) and head circumferences (R=0.640; P=0.000) (Table 2).
Discussion
Vit-D3 deficiency or insufficiency is prevalent in many countries and has been associated with various physiological disturbances and pathological outcomes. However, establishing direct relationships between Vit-D3 concentration in blood samples and the occurrence of diseases or health problems can be challenging because of the complex nature of Vit-D3 metabolism and its regulatory role in signaling pathways [23]. Discrepant results from clinical studies on the clinical outcomes of Vit-D3 arise from indirect or regulatory effects on metabolic pathways, epigenetic regulation, and ethnic differences in various studies worldwide. In the present study, we report the maternal and neonatal Vit-D3 blood levels in a group of pregnant mothers in central Iran who gave birth at our university hospital. Our results are in agreement with those of some published studies but in contrast to the findings of some others. Although such clinical data can help provide evidence, the precise clarification of Vit-D3’s role in specific metabolic, physiological, and anatomical/developmental events or processes requires basic studies. In the following section, we present the principal aspects of our study and discuss the possible causes of these clinical outcomes.
We evaluated 206 pregnant women, and with a cut-off point of 30 ng/mL for serum Vit-D3 levels, 83.5% of the participants had hypovitaminosis D3 (deficient or insufficient). Azami et al. [23] reviewed 18 studies, including 5,572 pregnant Iranian women, and reported that with a cut-off point of 30 ng/mL, 84.4% (95% confidence interval, 74.2–91.1%) of pregnant Iranian women had Vit-D3 deficiency. Therefore, at the time of our study submission (2022), the prevalence of hypovitaminosis D3 among pregnant women remained very high and unchanged. Only 16.5% of pregnant mothers had sufficient (>30 ng/mL) Vit-D3 levels. This finding suggests that Vit-D3 supplementation in pregnant women is an urgent objective that needs to be considered by health policymakers in Iran. We recommend that such a discussion be valid for most Middle Eastern countries, such as Iraq, Afghanistan, Pakistan, and Bahrain, as their lifestyle or economic status is similar to or even lower than that of the Iranian population. However, localized research is necessary for these countries to gain a clear perspective on the Vit-D3 status. In our study, the distribution rates of hypovitaminosis D3 among pregnant women were not dependent on educational level, location of habitation (rural or urban), or participant age. This finding suggests that the Vit-D3 status is not dependent on many demographic factors, particularly when it has a high prevalence, as observed in our study.
We measured Vit-D3 levels in UCB samples to determine the newborn Vit-D3 status. Using a cutoff point of 30 ng/mL, 84.7% of neonates had hypovitaminosis D3. Hosseinzadeh et al. [24] surveyed 151 pregnant women (serum samples) and 154 newborns (UCB samples) to determine 25-hydroxy vitamin D levels (cutoff=30 ng/mL). They have reported that the prevalence of vitamin D deficiency and insufficiency was 93.5% and 6.5% in pregnant women and 94.2% and 3.9% in newborns, respectively [24]. Our findings were similar to those of Hosseinzadeh et al. [24], confirming the dependence of newborn Vit-D3 levels on maternal resources. Therefore, we suggest Vit-D3 supplementation in pregnant women and regular monitoring, particularly during late pregnancy.
In our study, no significant difference in Vit-D3 levels was observed between male and female neonates or their mothers. Neonatal Vit-D3 levels were positively correlated with gestational age (R=0.161; P=0.022), indicating that older fetuses had higher levels of Vit-D3. This finding suggests that Vit-D3 may be transferred in greater amounts from the UCB to fetal circulation during the later stages of pregnancy. In addition, we observed a positive correlation between maternal and neonatal Vit-D3 levels. Mothers with higher Vit-D3 levels gave birth to neonates with higher UCB Vit-D3 levels. The strong association between maternal and neonatal Vit-D3 levels (R=0.744; P=0.000) provides evidence of the direct dependence of fetal Vit-D3 status on maternal Vit-D3 status. Hosseinzadeh et al. [24] have also reported a positive and significant correlation between Vit-D3 levels in mothers and newborns (R=0.913; P<0.001). Overall, a proper nutritional regimen or complementary formulations of Vit-D3 for mothers may alleviate Vit-D3 deficiency in pregnant women and their newborns.
In our study, maternal Vit-D3 levels were not significantly correlated with age but were close to being significant (R=0.131; P=0.062). However, newborn Vit-D3 levels were positively associated with maternal age (R=0.191; P=0.006), indicating that older mothers gave birth to newborns with higher Vit-D3 levels. This finding may be due to the higher storage of Vit-D3 in older mothers than in younger individuals in our study population. Additionally, older mothers had a higher BMI (R=0.219; P=0.003). Vit-D3 is a fat-soluble vitamin [2]. We hypothesized that mothers with a higher BMI may harbor higher amounts of non-circulating sources of Vit-D3 that could be available and transferred to the fetus during pregnancy. However, the association between Vit-D3 and the age of the participants is relatively rare and exists only for certain disease groups. For instance, Qahwaji [25] has reported a negative linear correlation between Vit-D3 and men with metabolic syndrome in men.
Our analysis of UCB samples revealed that they contained nontoxic concentrations of bilirubin and did not require hospitalization or intensive care. We observed that neonates with lower weights had higher UCB bilirubin levels and smaller head circumferences. However, neonatal Vit-D3 levels were not significantly correlated with UCB bilirubin or head circumference, and maternal Vit-D3 serum levels were not significantly correlated with UCB bilirubin or head circumference. Thus, we concluded that maternal Vit-D3 serum levels may not be reliable predictors or effectors of newborn bilirubin levels or scalp size. However, some studies have reported a negative correlation between hyperbilirubinemia and Vit-D3 levels in term newborns [13,15,26]. The discrepancy between our findings and those of these studies may be because of the differences in the populations studied. In our study, the mean±SD of UCB bilirubin concentration was 2.13±0.54 mg/dL (range, 0.4 to 4.5 mg/dL), whereas, in the study by Bhat et al. [14], the mean±SD of serum bilirubin was 18.05±1.09 mg/dL. In Mehrpisheh et al. [15] report, the mean±SD of indirect bilirubin was 17.12±1.92 mg/dL. Zia et al. [26] did not report a precise value in their published paper; they considered bilirubin >12 mg/dL as hyperbilirubinemia. Therefore, we did not observe newborns with severe hyperbilirubinemia. However, these studies have focused on hyperbilirubinemia in newborns and infants.
We monitored the hospitalization of the infants for the month following discharge by reviewing their records or conducting telephone calls. Our findings indicated that hyperbilirubinemia requiring hospitalization occurred independent of the mother’s Vit-D3 status. This suggests that mothers with deficient, insufficient, or sufficient Vit-D3 levels may give birth to newborns with severe hyperbilirubinemia days or months after hospital discharge.
In our study, maternal Vit-D3 levels did not significantly affect the occurrence of SGA, sepsis, IVH, death, or IUGR. However, other studies have reported associations between Vit-D3 levels and these variables. For example, Wang et al. [27] have reported that maternal Vit-D3 insufficiency (cut-off Vit-D3 <20 ng/mL) was independently associated with low birth weight and SGA in term infants. Noamam and Abdulla [28] found a significant association between Vit-D3 deficiency and low Apgar scores, low birth weight, small head circumference, and other outcomes. Boskabadi et al. [29] conducted a cross-sectional study and reported that 92.8% of infants with IVH had Vit-D3 <30 ng/mL, whereas this rate was 67% in infants without IVH.
Fig. 4 provides a graphical summary of the main findings of our study, highlighting the factors that affect fetal Vit-D3 levels and recommendations for addressing hypovitaminosis D3, including supplementation, drug-based formulations, and sunlight exposure. We believe that the indirect associations between Vit-D3 and the variables mentioned above may be attributed to differences in the study design, sample size, patient population, and other factors. However, these inconsistent results underscore the importance of conducting investigations using appropriate research methodologies. For instance, cross-sectional studies may not be suitable for identifying new risk factors and case-control models may be more appropriate.
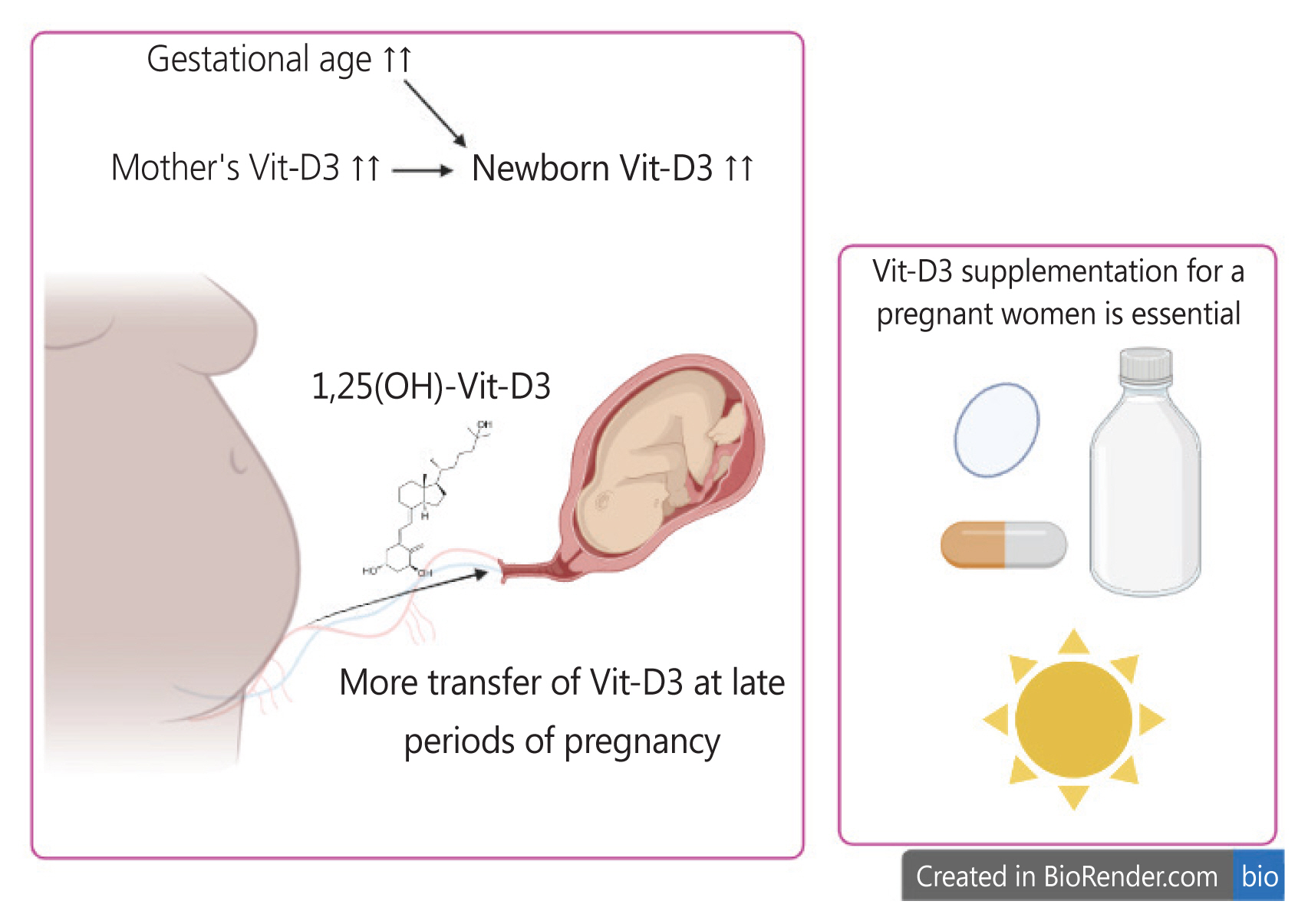
Major factors affecting fetal Vit-D3 levels in our study. Maternal Vit-D3 levels and gestational age have a direct impact on neonatal Vit-D3 levels. Therefore, we recommend Vit-D3 supplementation during pregnancy through nutritional regimens and sunlight exposure. Additionally, cesarean section should be postponed to a suitable time, as maternal Vit-D3 supplementation of the newborn occurs more effectively in the later stages of pregnancy. Vit-D3, vitamin D3;1,25(OH), 1,25-dihydroxyvitamin D.
The strength of our study lies in its assessment of the association between maternal and neonatal Vit-D3 levels across different gestational age groups as well as our recommendation for Vit-D3 supplementation during pregnancy. We believe that our findings will assist clinicians in developing effective strategies to prevent hypovitaminosis D3 in pregnant women and neonates.
In this study on a group of pregnant Iranian women, we found that maternal Vit-D3 levels provide the neonate with the necessary Vit-D3, particularly in the later stages of pregnancy. Furthermore, neonatal Vit-D3 levels were dependent on maternal age, likely due to higher BMI in older mothers. In our study, UCB bilirubin levels were not regulated by Vit-D3 levels but were higher in neonates with lower weight and smaller head circumference.
Overall, our results and those of other studies with similar or contrasting findings suggest that Vit-D3 supplementation during pregnancy is an urgent issue in midwifery and pregnancy care. We recommend that full-term babies have higher Vit-D3 levels than those born via cesarean section before term maturation. We propose that cesarean section should be postponed to a suitable time, as maternal Vit-D3 supplementation of the newborn occurs more effectively in the later stages of pregnancy. Even a one-day delay may help with better Vit-D3 supplementation by mothers. However, the metabolic, histological, and physiological effects of Vit-D3 may not be rapid enough for medical or clinical outcomes to manifest and be assessed using routine procedures. Investigating the effects of Vit-D3 on pregnancy physiology and toxicology would be valuable for researchers interested in the results of our study.
We evaluated 206 pregnant women and UCB samples from a single center. However, multicenter studies with larger sample sizes are preferable to better evaluate the association between maternal and neonatal Vit-D3 levels. Furthermore, controlling the inclusion and exclusion criteria in prospective studies can be challenging and requires collaboration between the clinical staff and researchers. Another limitation of our study was the Vit-D3 measurement method, which was an ELISA. It is recommended that this vitamin be measured using high-performance liquid chromatography (HPLC), which is not available in our clinical laboratory. The HPLC method has higher accuracy indices than immunoassay methods.
Notes
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the Ethics Committee of Arak University of Medical Sciences (approval number: IR.ARAKMU.REC.1399.032). Written informed consent was obtained from all the patients. All the experiments were performed in accordance with the Declaration of Helsinki.
Patient consent
The study aims were explained to all mothers in detail, and written informed consent was obtained.
Funding information
This study was financially supported by the Arak University of Medical Sciences (Grant ID:6095).