Feasibility of fetoscopic laser coagulation in triplet pregnancy
Article information
Abstract
Objective
To report the experiences of triplet pregnancies complicated by twin-to-twin transfusion syndrome (TTTS) treated with fetoscopic laser coagulation at a single center.
Methods
Herein, we conducted a retrospective analysis to investigate the management and perinatal outcomes of triplet pregnancies with TTTS treated at a single institution between 2017 and 2022.
Results
Seven of the 98 triplet pregnancies (7.1%) encountered were complicated by TTTS, and all were dichorionic triamniotic triplets. Of the seven triplet pregnancies complicated by TTTS, four were treated with fetoscopic laser coagulation at our center, at a median gestational age of 20 weeks. No procedure-related complications or maternal complications were observed. The survival rate was higher and perinatal outcomes were better in fetoscopic laser coagulation cases than in other management cases. Four donor and four recipient triplets survived, with a median gestational age of 33 weeks at delivery. Although there were no cases of poor neonatal outcomes, one case was diagnosed with white matter injury, suspected to be hypoxic-ischemic encephalopathy on postnatal investigation.
Conclusion
Fetoscopic laser coagulation is a feasible treatment option for triplet TTTS, provided the attending specialists have extensive experience with this technique.
Introduction
Fetoscopic laser coagulation (FLC) has emerged as one of the treatments of choice in twin to twin transfusion syndrome (TTTS). This procedure involves laser ablation of placental vascular anastomoses in monochorionic (MC) twin pregnancies. With the increasing rates of high-order multiple gestations, interest in the application of FLC for triplet pregnancies is increasing [1]. This raises the question of whether FLC is a superior therapeutic option for selective feticide use, especially in cases of severe TTTS in which all three fetuses could theoretically be salvaged. Despite this interest, the literature on FLC in triplets and higher-order multiple births with TTTS consists primarily of sporadic case reports and small series [2]. In this case series, we share our experience with triplet pregnancies complicated by TTTS and treated with FLC at a single center.
Materials and methods
We conducted a retrospective analysis of data on the management and perinatal outcomes of triplet pregnancies with TTTS treated at the Asan Medical Center in Seoul, Korea, between 2017 and 2022. We focused specifically on dichorionic (DC) triplet pregnancies, in which two fetuses shared one MC placenta and the third fetus had an independent single placenta. Chorionicity and amnionicity were established by sonographic examination during the first trimester of pregnancy, and confirmed by placental pathology after delivery. Detailed sonographic examinations were conducted on all fetuses, and were routinely performed on a weekly or biweekly basis at our center. We used one of two ultrasound machines, HERA W10 (Samsung Medison Co., Ltd., Seoul, Korea) or E10 Expert (General Electric Healthcare Austria GmbH & Co. OG, Pfaffing, Austria), both of which were equipped with a 2–6 MHz transabdominal probe, to perform all ultrasonographic measurements.
1. Diagnosis of TTTS
The diagnostic criteria for TTTS in triplet pregnancies were the same as those for twins, with the diagnosis based on sonographic findings of: 1) a maximum vertical pocket (MVP) of >8 cm in one sac (polyhydramnios) and 2) an MVP of <2 cm in the other sac (oligohydramnios) at any gestational age [3]. Once TTTS was diagnosed, the umbilical artery (UA), middle cerebral artery, and ductus venosus (DV) were evaluated in all fetuses using color and pulsed-wave Doppler to detect abnormal findings such as absent or reversed end-diastolic flow in the UA and reversed flow in the DV. Additionally, the left ventricular modified myocardial performance index (mod-MPI) was measured in all patients by positioning the Doppler sample volume in the internal leaflet of the mitral valve in an apical four-chamber view. The isovolumetric contraction time (ICT), relaxation time (IRT), and ejection times (ET) were measured using the valve click method. The mod-MPI was calculated as follows: (ICT+IRT)/ET [4].
2. Diagnosis of selective fetal growth restriction (sFGR)
The World Health Organization fetal growth charts (https://srhr.org/fetalgrowthcalculator/#/) were used to determine the weight percentile [5]. Discordance in the estimated fetal weight (EFW) was calculated by subtracting the EFW of the smaller twin from that of the larger twin, dividing by the EFW of the larger twin, and multiplying by 100. We defined sFGR as an EFW in the <10th percentile in one fetus with an intertwin EFW discordance of >25% [6].
3. Fetoscopic laser coagulation
FLC was performed in patients with TTTS at stage II or higher, symptomatic polyhydramnios, or cardiac dysfunction. Once the diagnosis of TTTS was confirmed, parents were provided counseling on the outcome, and offered various treatment options, including expectant treatment, serial amnioreduction, selective fetal termination, or FLC of the communicating placental vessels. After obtaining written informed consent from the parents, the procedure was performed by the same fetal therapy team, comprising two maternal-fetal medicine specialists (H.S.W. and M.Y.L.), three experienced nurses, and expert-trained assistants specializing in maternal-fetal medicine.
The operators used spinal anesthesia combined with a local lidocaine injection to perform FLC without intravenous sedation, enabling communication with the patient. The procedure was performed using a single 10-French flexible introducer inserted percutaneously into the recipient sac using the Seldinger technique. Depending on the placental location, a 2 mm fetoscope or 1.3 mm semi-rigid scope (Karl Storz GmbH, Tuttlingen, Germany) was passed through the cannula. Selective coagulation of all communicating vessels, including arteriovenous, arterio-arterial, and veno-venous anastomoses between the twins on the chorionic plate of the placenta, was performed using the diode laser at a power setting of 30 W with a 600 μm diameter laser fiber (WON TECH Co., Ltd, Seoul, Korea; or DiNonA Inc., Seoul, Korea). Amnioinfusion was performed with 0.9% normal saline to improve visualization during FLC, as necessary. Routine solomonization was performed, and the amniotic fluid was removed from the sac of the recipient at the end of the procedure. Transvaginal cervical cerclage was performed after FLC when the cervical length was less than 20 mm or the cervix was open.
4. Peri- and post-operative management
Perioperative management of patients who underwent FLC included prophylactic tocolytic agents and intravenous antibiotics (cephalosporins). Ultrasound examination was performed within 24 hours postoperatively, followed by daily ultrasound until the patient was discharged from the hospital. Weekly or biweekly follow-up ultrasonography was performed at our center. Complications such as intra-operative intrauterine bleeding, prelabor premature rupture of membranes (PPROM), chorioamnionitis, chorioamniotic membrane separation, recurrent TTTS, and post-operative twin anemia-polycythemia sequence (TAPS) were recorded. The decision to deliver was based on obstetric indications. Obstetric and neonatal outcome data were collected from the medical charts.
Results
1. FLC cases selection in triple pregnancies
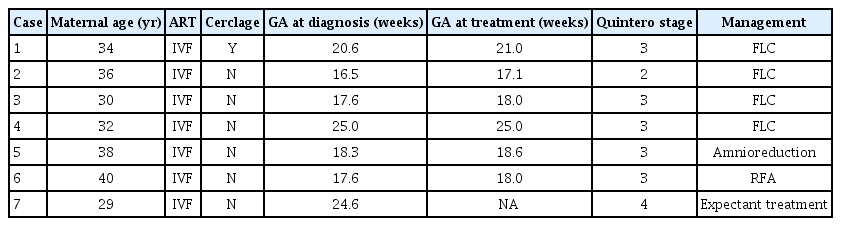
During the study period of 2017–2022, seven of 98 triplet pregnancies (7.1%) were complicated by TTTS, and all were dichorionic-triamnotic triplets. Of these, four patients underwent FLC, while the remaining three patients received amnioreduction, radiofrequency ablation (RFA), and expectant treatment (Fig. 1). Although all seven cases met the indications for FLC as they were stage II or higher, in three of these cases, FLC was not performed for the following reasons: in case no. 5, it was difficult to attempt FLC due to difficulties in surgical access because of the placental location, and only the recipient’s amnioreduction was performed. In case no. 6, due on the presence of 41% intertwin weight discordance with deterioration of Doppler findings in donor, RFA was performed on the donor. Case 7 was transferred to the recipient with fetal hydrops at a gestational age of 25 weeks, making it impossible to perform FLC. Details on the gestational age at the time of treatment and preoperative ultrasonographic findings in the seven TTTS-complicated cases are presented in Tables 1 and 2, respectively.
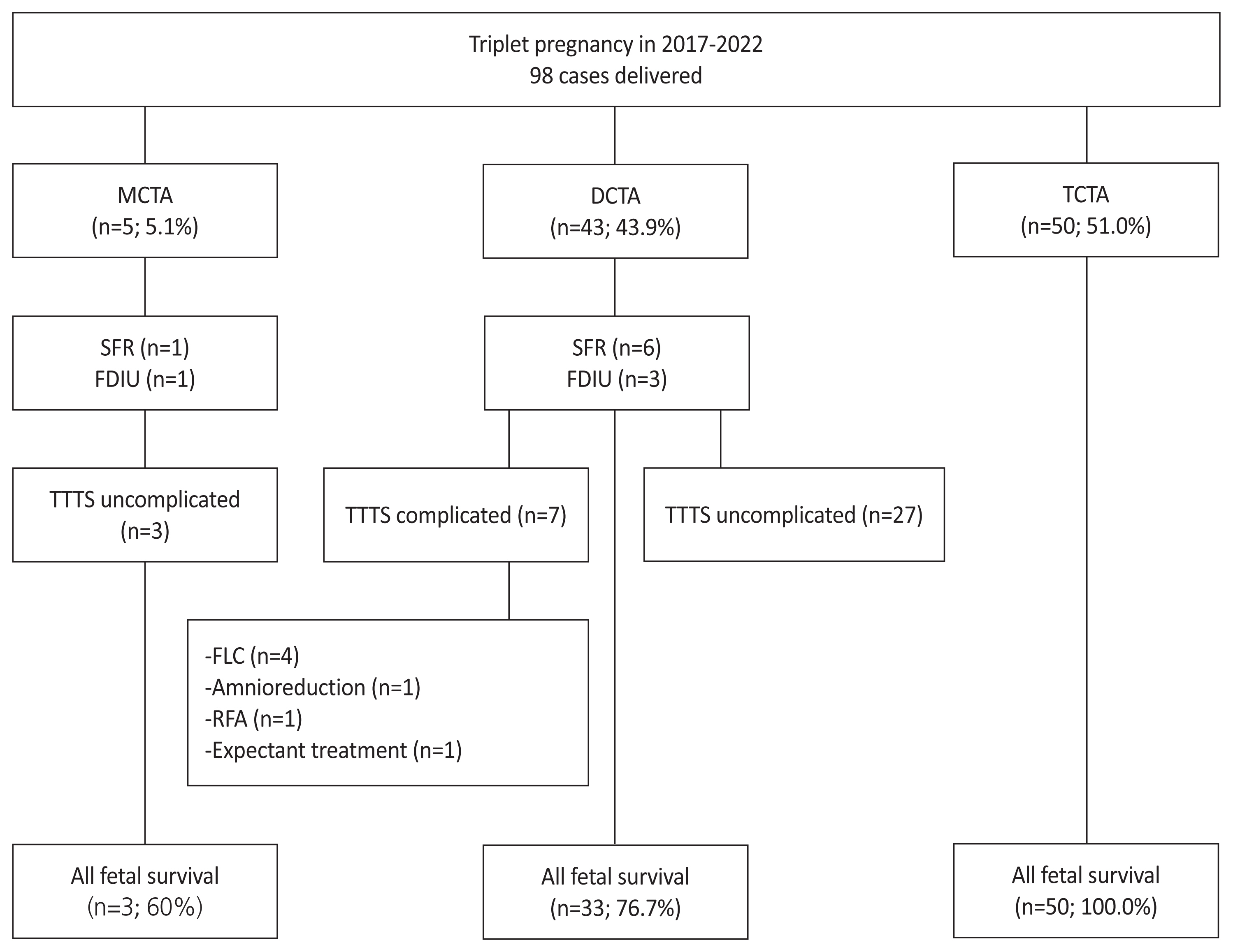
Flowchart illustrating the survival and management of chorionicity and amnionicity in triplet pregnancy. In our center, 7 of 98 triplet pregnancies (7.1%) were complicated by TTTS, and all were DCTA triplets. During the study period, four dichorionic triamniotic triplets underwent FLC at our center. MCTA, monochorionic-triamniotic; DCTA, dichorionic-triamniotic; TCTA, trichorionic-triamniotic; SFR, selective fetal reduction; FDIU, fetal death in utero; TTTS, twin to twin transfusion syndrome; FCL, fetoscopic laser coagulation; RFA, radiofrequency ablation.
2. Prenatal and procedure related characteristics of FLC cases
During the study period, four patients with DC triamniotic triplets underwent FLC at our center. Diagnosis was made at a median gestational age of 19 weeks, with one case classified as Quintero stage II and three cases as Quintero stage III; there were no stage IV cases. In case no. 2, although the recipient’s MVP was 69 mm, the quantitative measurement of amniotic fluid can be influenced by maternal position, fetal positioning, and other factors. Furthermore, typical features of TTTS, including the recipient’s enlarged bladder with cardiomyopathy and the donor’s invisible bladder with selective fetal growth restriction, were observed. Karyotyping was performed in one case, which revealed normal findings, and no sonographic structural anomalies were identified in any of the cases.
The mean interval between diagnosis and FLC was 1.2 days, and the mean procedure time was 41.7 minutes. No procedure-related complications such as recurrent TTTS or post-operative TAPS occurred, and no maternal complications such as hemoperitoneum or placental abruption were observed.
3. Perinatal outcomes of FLC cases
The survival rate and median gestational age at delivery were higher in FLC cases than in other management cases. In FLC cases, four donor and four recipient triplets survived, with a median gestational age at delivery of 33 weeks and a median birth weight of 1,753 g. In other management cases, two donor and two recipient triplets survived, with a median gestational age at delivery of 29.3 weeks and a median birth weight of 1,285 g.
PPROM occurred 72 days after FLC in only one patient (case no. 1). Otherwsie, all patients with FLC had an uncomplicated postpartum course, and pathological examination of the placenta after delivery revealed a DC triamniotic placenta with no vascular anastomoses between the donor and recipient (Fig. 2). In case no. 7, in which expectant treatment was administered, the recipient died within 2 days after birth. The median stay of neonates in the intensive care unit was lower in FLC cases (21.8 days vs. 47.5 days).

Photographs of the delivered placenta. There were no residual anastomoses between the recipient (fetus B) and donor (fetus C) in case no. 3. The territory of monochorionic placenta was successfully complete dichorionized by fetoscopic laser coagulation.
Three FLC cases were complicated by respiratory distress syndrome, and two had symptomatic patent ductus arteriosus requiring medical treatment. In one case, the recipient of case no. 1 was diagnosed with white matter injury, which was suspected as being hypoxic-ischemic encephalopathy (HIE) by brain ultrasonography (Fig. 3B). However, neurological symptoms were not observed until 20 months of age. None of the cases had poor neonatal outcomes, such as intraventricular hemorrhage (stage ≥III), necrotizing enterocolitis (stage ≥II), or moderate-to-severe bronchopulmonary dysplasia. The median follow-up duration was 21 months; seven patients showed normal growth and neurodevelopment, the recipient of case no. 4 exhibited delayed language development (Table 3).

Brain images the recipient of case no. 1 with a complicated cerebral injury. (A) Antenatal sonographic image at gestational age 28 weeks showing normal structures. (B) Postnatal sonographic image at 4 days of age showing multifocal echogenic foci (arrow) at the bilateral periventricular white matter, indicating white matter injury such as hypoxic-ischemic encephalopathy. (C) Postnatal brain magnetic resonance imaging at 1 month of age showing punctate white matter frontoparietal lesions (arrow) with hemorrhage showing as dark signal intensity on susceptibility weighted imaging.
Discussion
In this case series, we report the successful management of triplet pregnancies with TTTS using FLC. The survival rate was higher, and perinatal outcomes were better in FLC cases than in other management cases. Based on our experience, despite the small number of cases, we propose that FLC may be an effective therapeutic option for treating triplets.
The prevalence of TTTS in triplet pregnancies is currently unknown [1]. However, recent studies have indicated that TTTS occurs in 5% of DC triplet pregnancies and approximately 8% of MC triplet pregnancies [7,8]. These rates are not insignificant, especially considering that TTTS affects approximately 10–15% of all MC twin pregnancies and that triplet pregnancies have a higher incidence of intrauterine fetal death and miscarriage than twin pregnancies.
In addition to the limitations in surgical access, we found that the perinatal survival rates of at least one survivor in DC triplets were comparable to those in MC twins. Our data revealed that the donor and recipient fetuses survived, and the mean gestational age at delivery was 33 weeks. During the same period at our center, at least one survival and double survival rates of MC twins were 88.8% and 65.5%, respectively, and the mean gestational age at delivery was 32.5 weeks [9]. According to a recent systematic review, the overall fetal survival rate was 79% in DC triplets, compared to 74% in MC triplets [10]. Owing to the rarity of triplets, little is known about the comparison of perinatal outcomes among treatment options. Although the numbers were too small to draw conclusions, FLC is expected to increase survival rates in triplets, and result in a more advanced gestational age at birth compared with triplets treated with conservative methods.
Although the severe cerebral lesions were not encountered, one case was diagnosed with white matter injury suspected to be HIE by postnatal brain ultrasonography. However, no antenatal evidence of brain damage was found (Fig. 3A). Brain magnetic resonance imaging at 1 month of age confirmed the injury as a focal hemorrhage in the frontoparietal lobe (Fig. 3C). The incidence of severe cerebral lesions on cranial ultrasonography has been reported to range from 3% to 16% [11–14]. The exact mechanism responsible for antenatal cerebral injury in TTTS is unclear, as the methodologies and definitions vary. However, two main causes have previously been reported: antenatal injury resulting from hemodynamic imbalance, and postnatal injury resulting from prematurity. Spruijt et al. [15] compared the results of neonatal cranial ultrasonographic examinations in 267 neonates with TTTS treated with FLC, and concluded that prematurity was the only independent predictor of adverse neurodevelopmental outcome. Stirnemann et al. [16] further reviewed 1,023 cases of TTTS, and reported that TAPS, TTTS recurrence, and single intrauterine demise were all significantly associated with ischemic hemorrhagic brain lesions. In our cases, we assumed that the damage resulted from prematurity as the patient did not develop these complications after FLC, and the gestational age at delivery was the earliest among the patients. After FLC, close observation and re-evaluation should be performed to clarify the cause and onset. Additionally, long-term follow-up is necessary to determine whether the lesion will cause a significant neurodevelopmental delay, despite our patient not exhibiting neurological symptoms until 20 months.
Based on our data, FLC was performed in four of the seven triplets complicated by TTTS (57.1%). Although three of these patients had an anterior placenta, there were no operative complications. Since 2011, our center has conducted FLC for 262 cases, including triplets, averaging 26 procedures per year, which is considered sufficient for a large center based on a recent global survey [17]. FLC in DC triplets is generally considered technically more challenging than in twins because of the limitations in percutaneous access and anterior implantation of a single or fused placental mass, which may preclude adequate inspection of the vascular equator [18]. However, our data showed a mean procedure time of 41.75 minutes, which was not significantly longer than that of our previous twin study (29 minutes), suggesting that technical developments and an experienced team contributed to our successful outcomes [9].
Our analysis of fetal weight percentiles at diagnosis and birth revealed a converse relationship in the growth of recipients and donors, similar to our previously reported findings in MC twins, in which intertwin weight discordance decreased after FLC. These intertwin discordance changes showed the same tendency as those in a recent study [19]. However, the change in growth-restricted donors in our DC triplets was smaller than that in our MC twins with sFGR, likely because of the smaller placental territory of the growth-restricted fetus in triplets than in twins.
As triplet pregnancies are rare, whether the outcomes of laser surgery for placental anastomoses in triplet pregnancies complicated by TTTS are comparable to those in twin pregnancies has yet to be established. Large-scale studies reporting neonatal and maternal outcomes are warranted.
Notes
Conflict of interest
The authors have no potential conflict of interest relevant to this article to report.
Ethical approval
The study protocol was approved by the Institutional Review Board of Asan Medical Center (approval number. 2022-0536).
Patient consent
Informed consent was not required because of the retrospective nature of the study.
Funding information
None.