Outcomes of robotic sacrocolpopexy
Article information
Abstract
This review aimed to summarize the complications and surgical outcomes of robot-assisted sacrocolpopexy. Nineteen original articles on 1,440 robotic sacrocolpopexies were reviewed, and three systematic reviews and meta-analyses were summarized in terms of intraoperative, perioperative, postoperative, and/or surgical outcomes. Robotic sacrocolpopexy has demonstrated low overall complication rates and favorable surgical outcomes. Nevertheless, long-term follow-up outcomes regarding objective and/or subjective prolapse recurrence, reoperation rates, and mesh-related complications remain unclear. Further research is required to demonstrate whether the robotic approach for sacrocolpopexy is feasible or can become the modality of choice in the future when performing sacrocolpopexy.
Introduction
In 2014, Wu et al. [1] reported that the cumulative lifetime risk for pelvic organ prolapse (POP) surgery was 13%, and the age-specific annual risk for POP surgery increased progressively by the age of 80 years. They also reported that the number of women experiencing POP is expected to double by 2050 [1]. Despite advances in surgical techniques, the rate of reoperation after primary prolapse repair remains high and is estimated to be 10–30% [2,3]. Due to the high recurrence rate of conventional native tissue repair procedures [4], the use of sacrocolpopexy (SCP), which uses artificial meshes to suspend the vagina, is increasing worldwide [3,5]. Although SCP has proven to be the gold standard procedure for apical support due to its durability, its risks, such as high morbidity rates after a long operation time, chances of massive presacral bleeding when approached by laparotomy [6], and poor ergonomics and long learning curves of surgeons when performing laparoscopy [7], have made them reluctant to attempt SCP. However, the introduction of a robotic platform in SCP has changed the atmosphere [8,9]. The advantages of three-dimensional magnified vision, dexterity with multi-wristed instruments, surgeon comfort and ergonomics, and a shorter learning curve compared to the laparoscopic approach make it suitable for dissecting deep into the narrow field, mesh placement, and multiple intracorporeal suturing [7,8,10]. However, drawbacks, such as cost, lack of tactile sensation, and lack of high-quality evidence, should not be overlooked [7,8]. Although a few recent studies have emerged, long-term outcomes have not yet been thoroughly evaluated.
In this review, we aimed to describe the surgical techniques of robotic SCP under the da Vinci SP system® (Intuitive Surgical Inc., Sunnyvale, CA, USA) and to discuss the current evidence of surgical outcomes of robotic SCP.
Surgical techniques
Two types of da Vinci® surgical platforms (Intuitive Surgical Inc., Sunnyvale, CA, USA), da Vinci Xi® and da Vinci SP®, can be used for SCP; we mainly used the da Vinci SP® platform. The da Vinci SP® system, built for single-incision robotic surgery, allows the insertion of three instrument arms and a flexible endoscope camera through a single 2.5 cm cannula. This allows a narrow and deep access for surgeons to reach the pelvis. In addition, both the camera and the instruments have additional joints that allow for more degrees of freedom. In “cobra mode”, the camera is flexed 30° in the midline to allow an ideal view of the instruments, and camera angles can be adjusted further according to the surgeon’s needs. Although the flexible endoscope constrains the field of surgical view to be narrower, improved control of the optics compensates for these limitations. Additional joints in the instruments are also helpful for mesh fixation because precise control and versatility of movements at different angles and directions are required when suturing in a limited space.
For single-site robotic SCP under the da Vinci Si® and Xi® system, straight robotic arms were docked on four trocars through a single 2.5 cm umbilical incision. Three 8-mm cannulas were inserted into the umbilical glove port: one for the endoscope and the other for the docking of additional robotic arms. However, crowding and clashing among semi-rigid instruments inserted through separate curved cannulas, insufficient power, and limited degrees of freedom for precise instrument control during procedures present as challenges for this modality [11–13].
The surgical steps were as follows: first, all patients were prepared for lithotomy in a steep Trendelenburg position with a left table tilt of approximately 15°. The robotic patient cart was not located between the patient’s legs, allowing the assistant to place a uterine and/or vaginal manipulator during the procedure. The SP® trocar was placed in a multichannel single-incision port through a 2.5–3.0 cm vertical umbilical incision. A monopolar spatula or large needle driver was used for the right robotic arm (3 o’clock), Cadiere forceps for the left arm (9 o’clock), and fenestrated bipolar forceps for the centrally positioned arm (6 o’clock) (Fig. 1). Then, an accessory 5-mm trocar, serving as laparoscopic assistance, was inserted approximately 10–12 cm to the right of the umbilicus according to the complexity of the procedure, such as the presence of pelvic adhesions and/or obesity.
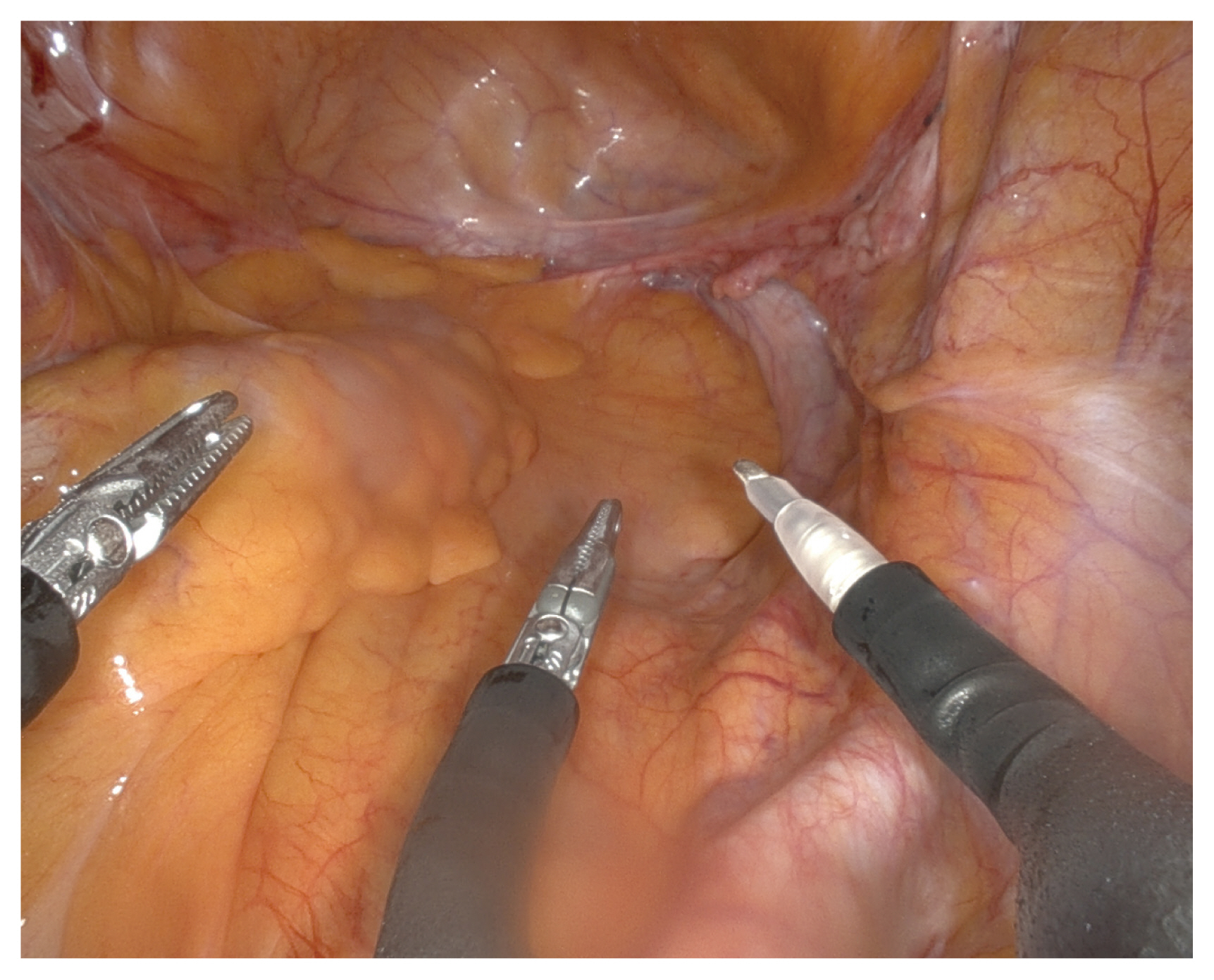
Insertion of instruments of the da Vinci SP® system. Monopolar spatula at the right-sided arm (3 o’clock), cadiere forceps for the left arm (9 o’clock), and the fenestrated bipolar forceps for the centrally positioned arm (6 o’clock).
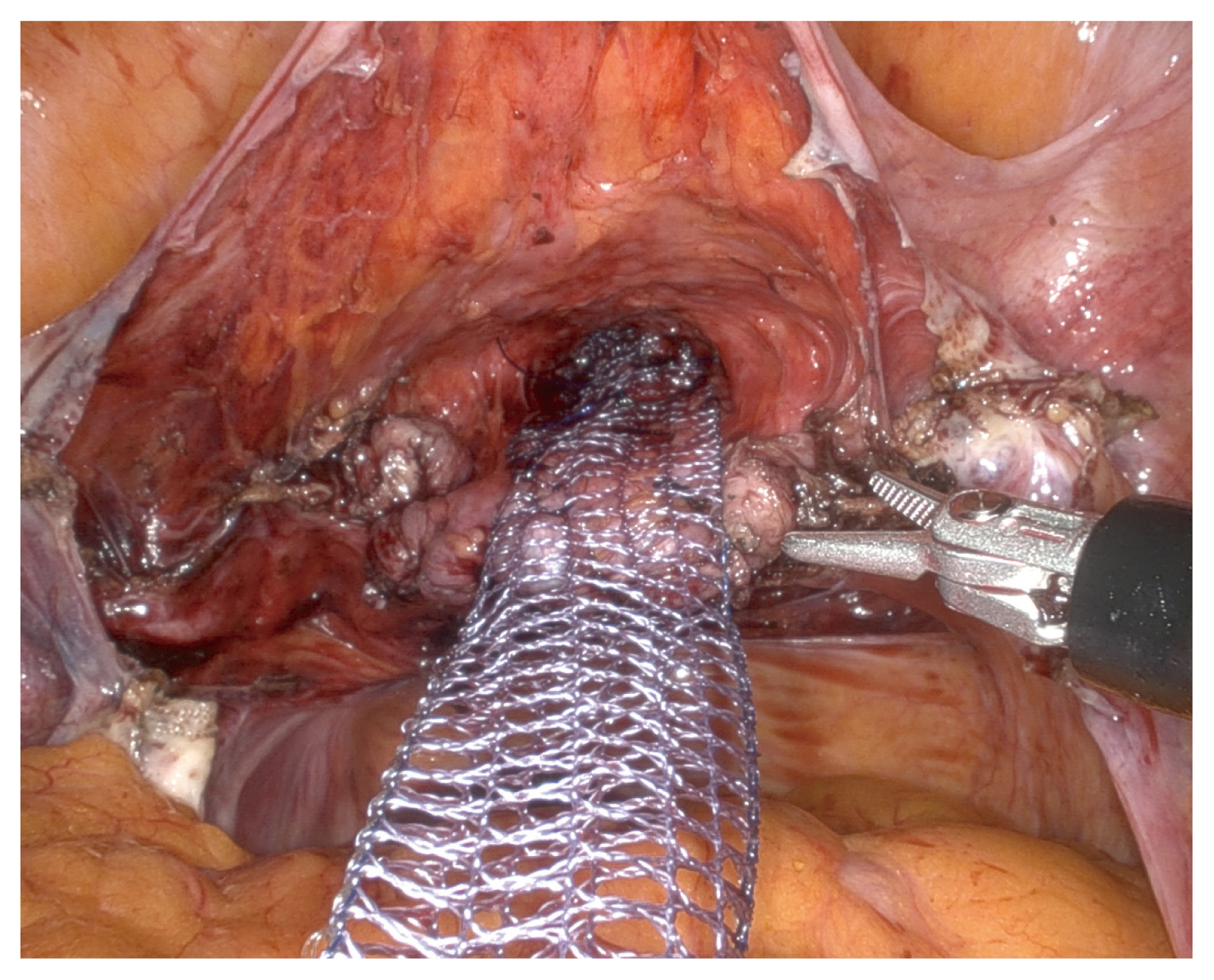
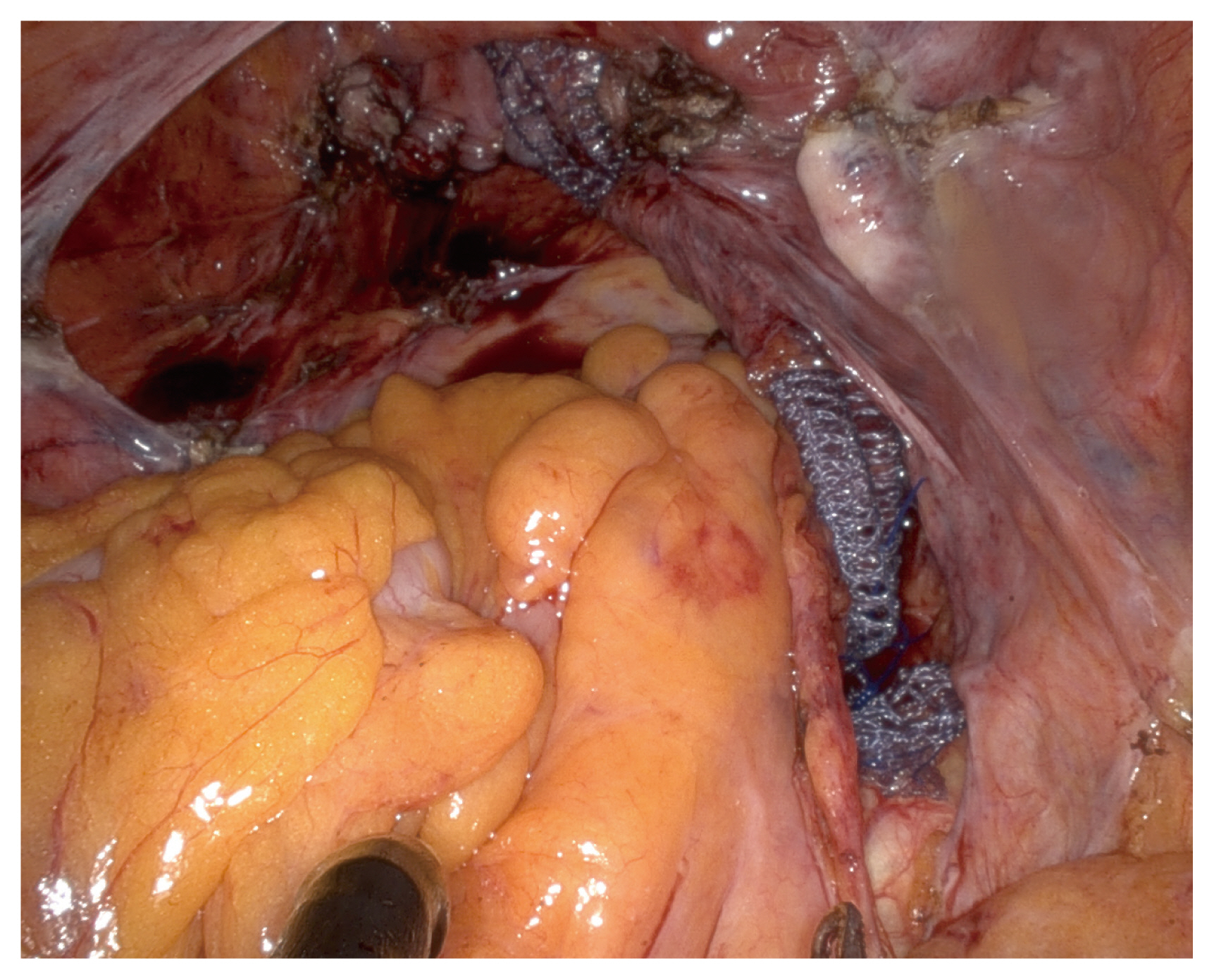
Briefly, an arrow-shaped dissection was performed to reveal the vesicovaginal space, possibly to the level near the perineal membrane (Fig. 2), and a V-shaped dissection along the rectovaginal space was performed as far as the perineal body was performed [14,15] (Fig. 3). While dissecting the rectovaginal space, the endoscope was manually rotated to set up an upward view. The retroperitoneal tunneling method was applied from the sacral promontory to the dissected pouch of Douglas [16] (Fig. 4). A partially absorbable type I polypropylene Y-shaped mesh was attached to the vaginal wall using absorbable sutures, and the tail end of the mesh was attached to the anterior longitudinal ligament of the S1 using nonabsorbable sutures. Finally, the peritoneum was approximated over the mesh [17].
Complications of robotic SCP
Intraoperative complications
1) Visceral injury
Cystotomy, during vesicovaginal dissection, is the most common intraoperative complication of SCP. In two systemic reviews and meta-analyses, the pooled estimates of the cystotomy rates during robotic SCP were 2.6% and 2.0%, respectively [10,18]. Interestingly, study cohorts consisting of post-hysterectomy vault prolapse showed higher cystotomy rates, reaching up to 12.5% [19–21], while the cohort mostly with supracervical hysterectomy (SCH) reported a lower cystotomy rate of 0–6% [13,22–25]. In a prospective study, Culligan et al. [22] reported no cystotomy in 316 robotic SCP cases, and the study population included approximately 75% of patients with concomitant SCH. Similarly, Mourik et al. [26] reported no cystotomy during 50 sacrohysteropexy procedures. However, two retrospective studies with a cohort of patients with post-hysterectomy vault prolapse only reported cystotomy rates of 11.1% and 12.5%, respectively [19,20]. Confounders may exist for the results since intraoperative visceral injuries largely depend on the surgeon’s experience, and the patient’s and operative characteristics. Nevertheless, caution is required when dissecting the vesicovaginal space of a post-hysterectomy vaginal vault. Once cystotomy occurs, one- or two-layered closure using absorbable sutures is performed, followed by a leakage test by instilling dye into the bladder. A catheter is then placed for 5–14 days, depending on the size and location of the injury. A cystogram is required before catheter removal, and reepithelialization of the bladder and restoration of its strength can be expected within 3–4 days and after 21 days, respectively. When a trigonal injury is suspected during the urogenital diaphragm approach, “call expert” and concerns regarding the ureter and/or urethra should be taken [27].
For intraoperative bowel injury, several studies have been conducted. Matthews et al. [28] reported two proctotomies out of 85 operations, and Germain et al. [29] reported one enterotomy out of 52 cases, whereas Anand et al. [20] reported four cases of bowel injury among 50 robotic SCP to correct post-hysterectomy vaginal vault prolapse. Two recent randomized studies by Illiano et al. [30] and Matanes et al. [13] showed no bowel injuries in 113 robotic SCP. Two systematic review articles reported rates <1% [10,18].
The vaginotomy rate was consistent with that of other visceral injuries. Most articles demonstrated zero or one vaginotomy, whereas one study involving post-hysterectomy vault prolapse reported a rate as high as 24% (11/45) [20].
2) Presacral bleeding
Dissection of the sacral region for mesh fixation is considered the most critical step during the SCP procedure. Disruption of the middle sacral vessels, sacral venous plexus, and/or left common iliac vein can cause drastic hemorrhage. Thus, identification of these vessels and caution regarding their anatomical variations during the procedure are important. According to a comprehensive review of abdominal SCP by Nygaard et al. [6], the incidence of hemorrhage or transfusion during SCP is 4.4%, with some cases arising from presacral hemorrhage. Few studies on robotic SCP have included presacral bleeding as an intraoperative complication. van Zanten et al. [23] reported one presacral bleeding out of 130 robotic SCP cases in their prospective study, whereas Anand et al. [20] found two presacral hemorrhages out of 50 robotic SCP operations in their retrospective cohort. Both studies reported that the surgical route was changed due to presacral bleeding. Anger et al. [31] presented one left iliac venotomy in 40 robotic SCP cases that closed intraoperatively without changing the access route. The risk of presacral hemorrhage during robotic SCP is quite low and is estimated to be lower than that associated with the conventional abdominal approach. Optimal magnification with a three-dimensional view and multi-articulated instruments that allow fine dissection to distinguish adjacent structures may have contributed to the decreased risk of accidental injury to the surrounding vessels.
Perioperative outcomes
We defined perioperative outcomes using estimated blood loss (EBL), operation time (from incision to closure), and length of hospital stay, which reflect the safety or short-term morbidity of the surgery. Most studies demonstrated that the average EBL during robotic SCP is minimal (range 50–250 mL), and the length of hospital stay ranges from 1 to 7 days, with an average of 2 days [10,18]. Although a few studies have reported console time, we listed the total operation time (from skin incision to closure) to evaluate the perioperative outcome of robotic SCP, which appears consistently in the literature. A systematic review by Hudson et al. [18] showed that the weighted average total operative time was approximately 230 minutes, and another systematic review presented a median of 194 minutes [10]. Recently, robotic SCP using a single-incision (both single-site and single-port) technique reported a total operation time of less than 200 minutes [13,17,24,25]. This does not mean that single-incision SCP takes less time, since operative time is influenced by many factors, such as the surgeon’s proficiency; thus, the total operation time cannot be the sole indicator for the index surgery. However, easier docking system of SP® platform may have contributed to shorter total operation time.
Mesh-related complications
Mesh-related complications are a specific issue in SCP, and smoking, chronic steroid use, mesh type, and concurrent hysterectomy are known risk factors for mesh-related complications [2,32,33].
Siddiqui et al. [34] observed a total mesh erosion rate of 4.3% (3/70) and no mesh erosion among concomitant SCH groups during an 18-month mean follow-up period. The study by Mourik et al. [26] which consisted of 50 uterus-preserving sacrohysteropexy cases, reported no mesh complications, and a study by Chan et al. [19] whose inclusion criterion was post-hysterectomy vault prolapse, showed similar results (0/16). In contrast, Geller et al. [35] demonstrated a 13.3% (2/15) mesh erosion rate in their prospective non-comparative study, with 75% of patients undergoing total hysterectomy. However, in a retrospective study comparing robotic SCP with total hysterectomy and SCH, the two groups demonstrated insignificant differences in the mesh erosion rate at the 1-year follow-up (7.5% vs. 2.3%; P=0.35) [36].
Currently, most studies have reported mesh-related outcomes with the application of a type I (macroporous) lightweight monofilament polypropylene mesh, which has a lower mesh complication rate [6,33]. All the aforementioned studies used this type of mesh.
Several reviews have reported SCP mesh erosion rates, and the results were comparable between different access modalities. Traditionally, in a comprehensive review of abdominal SCP, the risk of mesh erosion was 3.4%, and polypropylene meshes were reported to have a lower incidence at 0.5% [6]. A recent review of mesh exposure after minimally invasive SCP, including either laparoscopic or robotic SCP, indicated that the overall rate of mesh exposure was 3.5% [33]. In two systematic reviews of robotic SCP, the pooled estimates of mesh erosion rates were 2.0% and 4.1%, respectively [10,18].
An extended Colpopexy and urinary reduction efforts trial, which represented a long-term 7-year follow-up outcome of abdominal SCP, showed that the occurrence of mesh erosion tends to increase over time, reaching 10.5% [37]. This risk can change as more evidence of robotic SCP accumulates. Nevertheless, the incidence of mesh exposure after robotic SCP is low (2–4%) based on the currently available data. Furthermore, studies reporting mesh erosion rates demonstrate much fewer cases of reoperation for mesh complications; the most common site for mesh exposure is the vagina, and most cases can be managed conservatively with topical estrogen without surgical intervention.
Surgical outcomes
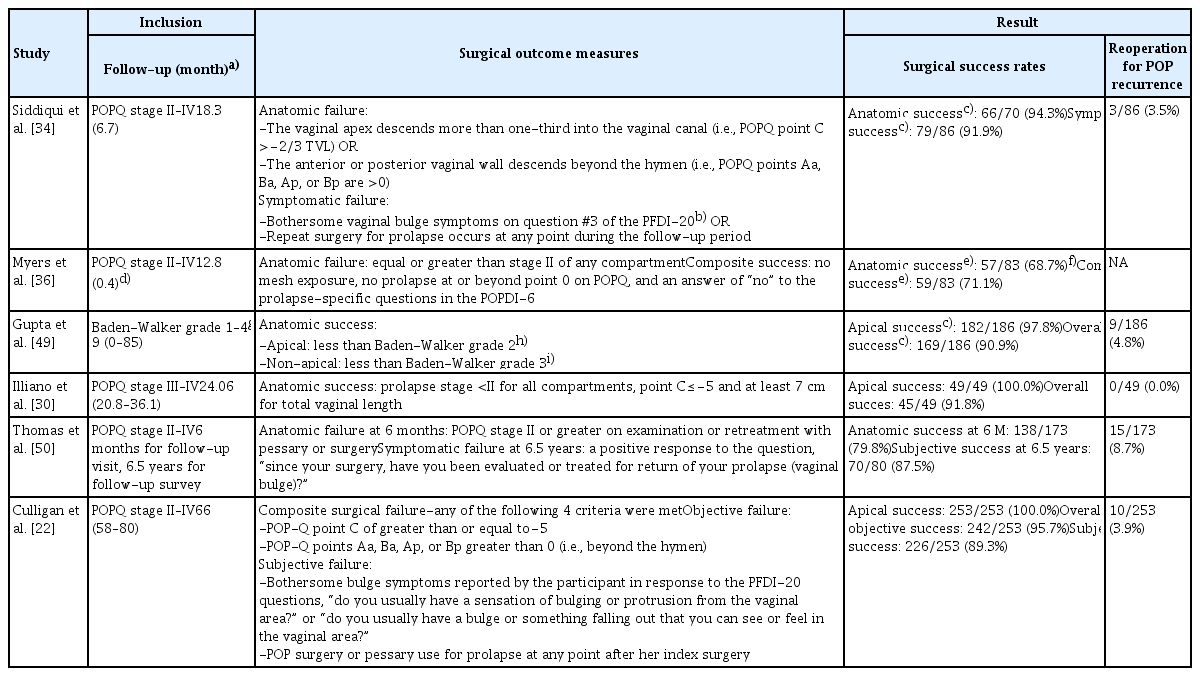
A meta-analysis by Hudson et al. [18] for the surgical outcomes of robotic SCP yielded a combined estimated apical success rate of 98.6% (95% confidence interval [CI], 97.0–100.0%) with the definition of apical prolapse as less than or equal to pelvic organ prolapse quantification stage I. Notably, they reported that the reoperation rate for recurrent prolapse was 3.3%, and the reoperation rates for recurrent apical and non-apical prolapse were 0.8% and 2.5%, respectively. The majority of reoperations were posterior colporrhaphies. Another meta-analysis demonstrated that the overall objective cure rate, irrespective of the vaginal compartment, ranges from 84% to 100% [10]. Although the criteria used to determine surgical success or failure vary wherein even apical and non-apical prolapses are defined differently within the studies, the overall success rate for robotic SCP is promising (Table 1).
In general, risk factors for surgical failure after pelvic reconstructive surgery include younger age, obesity, family history, and advanced prolapse stage [2,38–40]. In terms of SCP, the approaches (abdominal vs. laparoscopic vs. robotic), types of mesh, and mesh anchoring methods (absorbable vs. nonabsorbable sutures, interrupted vs. barbed running sutures, and anchor vs. suture) were suggested as possible factors for surgical failure. However, corresponding randomized studies have proven that none of these factors were significant [21,31,41–46]. Further research is required to determine the existence of robotic SCP-specific predictors of prolapse recurrence. To date, differences in surgical outcomes between multiport, single-site, and single-port robotic approaches showed insignificant results [13,17,24,25].
Given that a paramount of prolapse repair is the restoration of quality of life, studies presenting their outcomes by applying composite measures of success are on the rise (Table 1). The subjective success rate measured through structured queries also showed promising outcomes, significantly improving prolapse and urinary, defecatory, and sexual functions with a high satisfaction rate.
Similar to the mesh-related problems, prolapse recurrence can increase over time. Thus, long-term data collection of patients undergoing robotic SCP is required.
Recently, two studies comparing the outcomes of single-site and single-port robotic SCP, which included 127 and 74 cases, respectively, were published [17,25]. Although evidence is limited, the two modalities seem to be comparable in terms of intraoperative, perioperative, postoperative, and surgical outcomes. Additional joints of the instruments including the endoscope are the advantage of SP® system, which can reach deep into the urogenital diaphragm from the sacral promontory, while single-site system faces setbacks by crowding and clashing among the semi-rigid instruments resulting to a limited range of motion.
Conclusion
The purpose of this review was not to determine the superiority or inferiority of robotic SCP over other modalities of SCP. This review is written in a noncomparative and narrative manner with several outstanding studies on robotic SCP so that readers can collectively overview the data regarding robotic SCP in various aspects at this time point. This review also comes from the limited reports on single-incision robotic SCP. To the best of our knowledge, most of the related articles deal mainly with surgical techniques and tips regarding their initial experience of single-incision robotic SCP [12,16,47,48]. For this reason, comparing outcomes of robotic SCP among multiport, single-site, and single-port approaches seem undesirable; however, it may still possess future research value.
Nonetheless, the overall low intraoperative, perioperative, and postoperative complication rates reflect that robotic SCP can be performed safely, and has a promising surgical success rate. In addition, most of the comparative studies quoted above have proven the non-inferior surgical outcomes of robotic SCP compared to laparotomy or laparoscopic access. Therefore, surgeons dealing with the pelvic organ prolapse should consider the advantages of the robotic approach for SCP, which can be performed safely and effectively. Currently, research is emerging on single-incision robotic SCP, particularly when performed using a single-port robotic platform [17,24,25,48]. The specific complications and surgical outcomes of single-port robotic SCP should be discussed in the near future.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
Institutional Review Board (IRB) approval is not applicable for this study.
Patient consent
There is no need for patient consent in this review article.
Funding information
None.