 |
 |
- Search
| Obstet Gynecol Sci > Volume 66(5); 2023 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
Notes
Ethical approval
This study was approved by the Institutional Review Board of Severance Hospital (IRB No. 4-2018-0747), performed in accordance with the principles of the Declaration of Helsinki.
Funding information
This study was financially supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2022R1A2C2008378), Faculty Research Grants of Yonsei University College of Medicine (6-2017-0189), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health & Welfare, Republic of Korea (HI18C2047).
Fig. 1
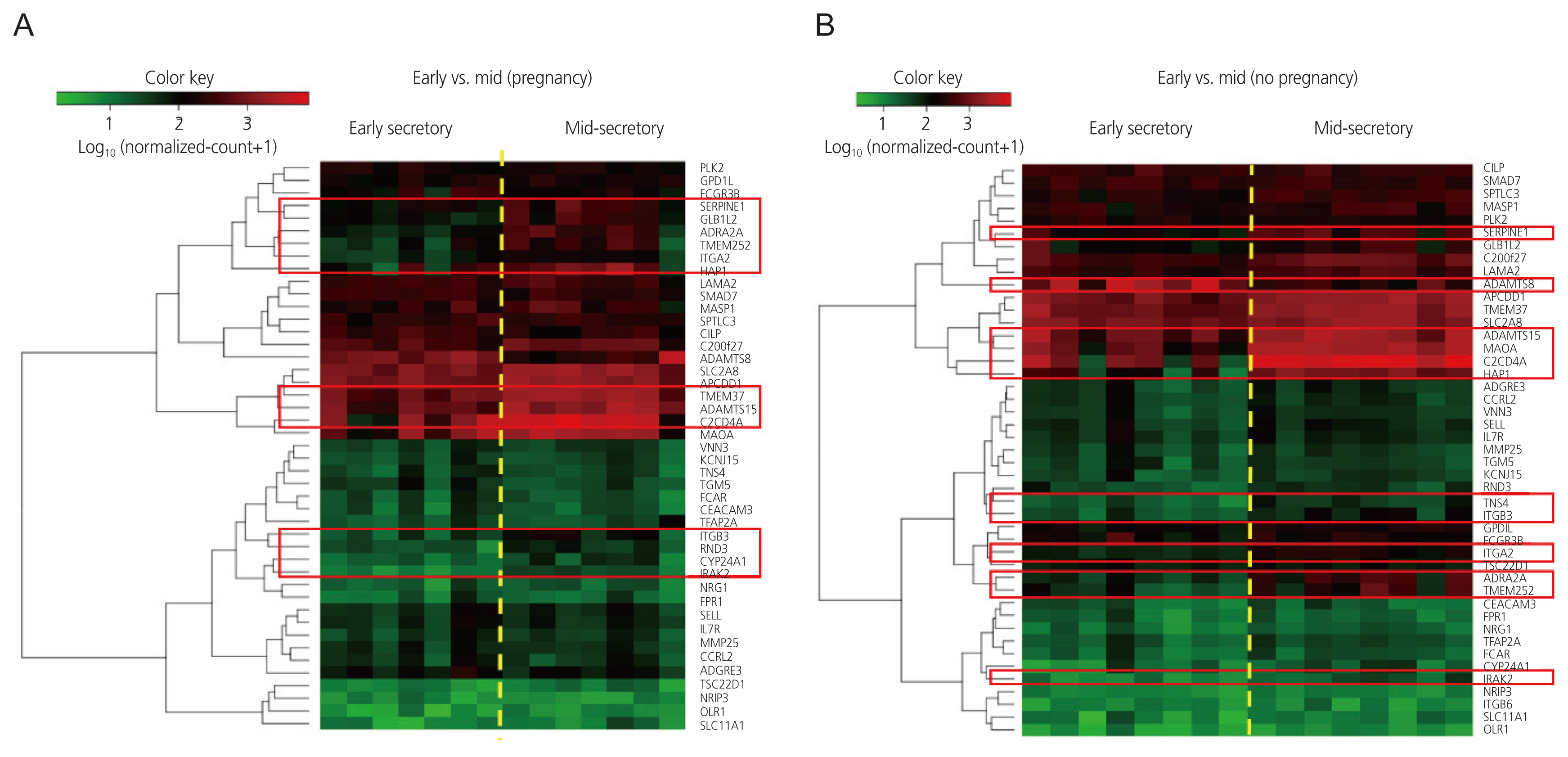
Fig. 2

Table 1
Table 2
| Gene name | Fold change | Statusa) | P-value | Gene description |
|---|---|---|---|---|
| ADRA2A | 5.15 | Up | 8.55×10−6 | Adrenoceptor alpha 2A (source: HGNC symbol; acc: HGNC: 281) |
| IRAK2 | 6.54 | Up | 1.45×10−6 | Interleukin 1 receptor-associated kinase 2 (source: HGNC symbol; acc: HGNC: 6,113) |
| TMEM37 | 2.55 | Up | 0.000748 | Transmembrane protein 37 (source: HGNC symbol; acc: HGNC: 18,216) |
| ADAMTS15 | 2.81 | Up | 0.00119 | ADAM metallopeptidase with thrombospondin type 1 motif 15 (source: HGNC symbol; acc: HGNC: 16,305) |
| GLB1L2 | 3.47 | Up | 0.00124 | Galactosidase beta 1 like 2 (source: HGNC symbol; acc: HGNC: 25,129) |
| SERPINE1 | 2.86 | Up | 0.00173 | Serpin family E member 1 (source: HGNC symbol; acc: HGNC: 8,583) |
| ITGB3 | 3.69 | Up | 0.00222 | Integrin subunit beta 3 (source: HGNC symbol; acc: HGNC: 6,156) |
| TMEM252 | 3.85 | Up | 0.00358 | Transmembrane protein 252 (source: HGNC symbol; acc: HGNC: 28,537) |
| RND3 | 2.53 | Up | 0.00901 | Rho family GTPase 3 (source: HGNC symbol; acc: HGNC: 671) |
| HAP1 | 3.77 | Up | 0.0111 | Huntingtin-associated protein 1 (source: HGNC symbol; acc: HGNC: 4,812) |
| CYP24A1 | 3.41 | Up | 0.0195 | Cytochrome P450 family 24 subfamily A member 1 (source: HGNC symbol; acc: HGNC: 2,602) |
| C2CD4A | 3.33 | Up | 0.0227 | C2 calcium-dependent domain containing 4A (source: HGNC symbol; acc: HGNC: 33,627) |
| ITGA2 | 2.58 | Up | 0.0249 | Integrin subunit alpha 2 (source: HGNC symbol; acc: HGNC: 6,137) |
ADRA2A, adrenoceptor alpha 2A; HGNC, HUGO Gene Nomenclature Committee; IRAK2, interleukin 1 receptor-associated kinase 2; TMEM37, transmembrane protein 37; ADAMTS15, a disintegrin and metalloproteinase with thrombospondin type 1 motifs, 15; GLB1L2, galactosidase beta 1 like 2; SERPINE1, serpin family E member 1; ITGB3, integrin subunit beta 3; TMEM252, transmembrane protein 252; RND3, Rho family GTPase 3; HAP1, huntingtin associated protein 1; CYP24A1, cytochrome P450 family 24 subfamily A member 1; C2CD4A, C2 calcium dependent domain containing 4A; ITGA2, integrin subunit alpha 2.
Table 3
| Gene name | Fold change | Statusa) | P-value | Gene description |
|---|---|---|---|---|
| ITGA2 | 2.84 | Up | 2.96×10−7 | Integrin subunit alpha 2 (source: HGNC symbol; acc: HGNC: 6,137) |
| ADAMTS8 | −2.82 | Down | 5.69×10−6 | ADAM metallopeptidase with thrombospondin type 1 motif 8 (source: HGNC symbol; acc: HGNC: 224) |
| MAOA | 4.36 | Up | 1.63×10−5 | Monoamine oxidase A (source: HGNC symbol; acc: HGNC: 6,833) |
| C2CD4A | 6.29 | Up | 8.41×10−5 | C2 calcium-dependent domain containing 4A (source: HGNC symbol; acc: HGNC: 33,627) |
| ADRA2A | 4.34 | Up | 0.000375 | Adrenoceptor alpha 2A (source: HGNC symbol; acc: HGNC: 281) |
| IRAK2 | 3.37 | Up | 0.00113 | Interleukin 1 receptor-associated kinase 2 (source: HGNC symbol; acc: HGNC: 6,113) |
| ITGB3 | 2.90 | Up | 0.00326 | Integrin subunit beta 3 (source: HGNC symbol; acc: HGNC: 6,156) |
| ADAMTS15 | 2.08 | Up | 0.0112 | ADAM metallopeptidase with thrombospondin type 1 motif 15 (source: HGNC symbol; acc: HGNC: 16,305) |
| TMEM252 | 4.08 | Up | 0.0123 | Transmembrane protein 252 (source: HGNC symbol; acc: HGNC: 28,537) |
| SERPINE1 | 2.07 | Up | 0.0124 | Serpin family E member 1 (source: HGNC symbol; acc: HGNC: 8,583) |
| HAP1 | 2.94 | Up | 0.0155 | Huntingtin associated protein 1 (source: HGNC symbol; acc: HGNC: 4,812) |
ITGA2, integrin subunit alpha 2; HGNC, HUGO Gene Nomenclature Committee; ADAMTS8, a disintegrin and metalloproteinase with thrombospondin type 1 motifs, 8; MAOA, monoamine oxidase A; C2CD4A, C2 calcium dependent domain containing 4A; ADRA2A, adrenoceptor alpha 2A; IRAK2, interleukin 1 receptor associated kinase 2; ITGB3, integrin subunit beta 3; ADAMTS15, a disintegrin and metalloproteinase with thrombospondin type 1 motifs, 15; TMEM252, transmembrane protein 252; SERPINE1, serpin family E member 1; HAP1, huntingtin associated protein 1.
Table 4
KEGG, Kyoto encyclopedia of genes and genomes; TMEM37, transmembrane protein 37; GLB1L2, galactosidase beta 1 like 2; RND3, Rho family GTPase 3; GTP, guanosine diphosphate; CYP24A1, cytochrome P450 family 24 subfamily A member 1; RNA, ribonucleic acid; MAOA, monoamine oxidase A; HAP1, huntingtin associated protein 1; GABA, gamma-aminobutyric acid; ADAMTS8, a disintegrin and metalloproteinase with thrombospondin type 1 motifs, 8.





















