Body fat distribution and insulin resistance among Korean middle-aged women: a Korean National Health and Nutrition Examination Survey
Article information
Abstract
Objective
To evaluate menopause-related changes in body fat distribution and their relationship with insulin resistance in middle-aged Korean women.
Methods
We analyzed women aged 40–60 years using data from the National Health and Nutrition Examination Survey conducted from 2008 to 2011. Body fat was measured using dual-energy X-ray absorptiometry. Insulin resistance was assessed using the homeostasis model assessment of insulin resistance (HOMA-IR).
Results
Among 3,468 participants, menopausal women (n=1,489) had a higher body mass index (BMI) and higher trunk, arm, and head fat percentages than premenopausal women (n=1,979). However, no significant difference was found in the leg fat percentage according to menopausal status. Multivariable regression analysis for HOMA-IR showed that trunk fat percentage, BMI, and waist circumference positively correlated with insulin resistance and leg fat percentage negatively correlated after adjusting for several confounding factors, whereas menopausal status was not associated with HOMA-IR.
Conclusion
Middle-aged women not only have different body weights and BMI but also have different body fat distributions according to menopausal status. Each fat percentage change in the trunk and leg is differently associated with metabolic health, particularly insulin resistance. To evaluate the metabolic health of middle-aged women, BMI is generally noted; however, body fat distribution, which can be easily assessed using dual-energy X-ray absorptiometry, should also be considered.
Introduction
Menopause is defined as the permanent cessation of menstrual periods resulting from the loss of ovarian follicles, along with serum estrogen deficiency. The average age of menopause is between 46 and 52 years, but it differs by race [1]. Several previous studies have shown that the mean menopausal age varies from 46.9 to 50.4 years in Korean women [2,3]. Both menopause and its accompanying sex hormone changes have been reported to be related to several metabolic changes, including the prevalence of obesity, dyslipidemia, insulin resistance, type 2 diabetes, and increased risk of cardiovascular disease [4,5]; however, the exact mechanisms underlying these associations are not yet fully understood. Thus, it is important to monitor and manage high-risk groups for chronic diseases in middle-aged women.
Previous studies have presented conflicting results on whether menopause is directly related to obesity or body fat distribution in middle-aged women. A recent systemic review proposed that the change in fat mass quantity between pre-menopausal and postmenopausal women was predominantly attributed to increasing age, rather than the menopausal transition itself [6]. Conversely, several studies have suggested that a decrease in endogenous estrogen levels during menopause may increase the total amount of overall body fat, particularly central fat, also referred to as trunk or torso fat, in postmenopausal women [7–10]. To clarify the factors associated with body fat distribution, further research using more accurate measurements such as dual-energy X-ray absorptiometry (DEXA) or computed tomography (CT), rather than anthropometric measurements such as body mass index (BMI) or waist circumference, is needed [11].
Central or visceral obesity, prevalent among menopausal women, has been recognized as a strong predictor of insulin resistance, hyperinsulinemia, type 2 diabetes, and cardiovascular risk [12–14]. However, most previous studies have been based on anthropometric measurements, and how specific changes in body fat distribution in each body part after menopausal transition are related to cardiometabolic disorders, including insulin resistance, remains unclear.
Therefore, this study aimed to investigate body fat distribution according to menopausal status and its relationship with insulin resistance among middle-aged Korean women using nationwide population-based data.
Materials and methods
1. Data collection and study participants
This study used data from the Korea National Health and Nutrition Examination Survey (KNHANES), a nationally representative, cross-sectional, population-based survey of health and nutritional status conducted by the Korea Centers for Disease Control and Prevention. This study used data from the seventh KNHANES (2008–2011), which included items on socioeconomic status and women’s health.
In the KNHANES database, we analyzed middle-aged women aged 40–60 years who completed the health examination survey, including body fat measurement (total fat mass, fat percentage, and body fat percentage) based on the results of DEXA and menopausal history. The exclusion criteria were as follows: patients with myocardial infarction, stroke, kidney failure, and history of malignant disease (gastric cancer, colon cancer, breast cancer, cervical cancer, etc.) and those who had surgically or artificially induced menopause (hysterectomy).
2. Measurement of body fat distribution
Body composition was analyzed using DEXA using a Discovery fan beam densitometer (Hologic Inc., Bedford, MA, USA). DEXA is a noninvasive technique for measuring bone mineral density using spectral imaging and body composition analysis, which allows the regional assessment of the proportion of fat and non-fat tissue. The fat mass and percentage of each part of the body were measured using DEXA during health examinations. Using the software, the body was divided into six parts (head, trunk, left and right arm, and left and right leg). Arm and leg fat were defined as the sum of the fat in the two arms and legs, both divided by two.
3. Definitions
Menopause was defined as the cessation of menstruation for more than 12 months. The survey was conducted through face-to-face interviews conducted by trained staff in the KNHANES. Menopausal status was determined based on self-reports and categorized as either premenopausal or post-menopausal. BMI is a person’s weight in kilograms divided by the square of the height in meters. Body fat percentage is the total fat mass divided by the total body mass multiplied by 100. Insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR). HOMA-IR has been widely used to measure insulin sensitivity based on fasting plasma glucose and insulin concentration [15].
Participants responded to a survey on lifestyle and demographic factors, including smoking status, drinking status, physical exercise level, household income level, and education level. Education level was divided into three categories: elementary school or no education, middle or high school graduates, and university graduates. To calculate the house-hold income level, the mean monthly household income was divided by the root of the number of household members and classified into quartiles (from lower to higher). Moderate-intensity exercise was defined as a more exertive physical activity than usual for a minimum of 30 minutes a day for 5 days in a week, or high-intensity exercise for at least 20 minutes per day for 3 days in a recent week.
4. Statistical analyses
Continuous variables are expressed as mean±standard deviation, unless specified otherwise. Statistical comparisons were conducted using the t-test for continuous variables and Pearson’s chi-squared test for categorical variables. Multivariable regression analysis was performed. P-values <0.05 were considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 3,468 Korean women aged 40–60 years were included in this study: 1,979 premenopausal women and 1,489 postmenopausal women. A comparison of the baseline characteristics according to menopausal status is shown in Table 1. The mean ages of the premenopausal and postmenopausal women were 45.21±3.81 years and 54.76±3.55 years, respectively. Postmenopausal women have higher parity, BMI, waist circumference, and prevalence of chronic diseases, more frequent use of oral contraceptive and menopausal hormone therapy, lower education levels, less intensive physical activity, higher carbohydrate and lower fat uptake, higher blood pressure, higher fasting glucose levels, and generally worse lipid profiles than premenopausal women. Total fat mass was significantly higher in postmenopausal women than that in premenopausal women (20.08±4.92 kg and 19.37±4.89 kg, respectively, P<0.001). The trunk, arm, and head fat percentages were significantly higher in postmenopausal women than in premenopausal women (Fig. 1). However, no significant difference was observed in the leg fat percentage according to menopausal status among middle-aged women.
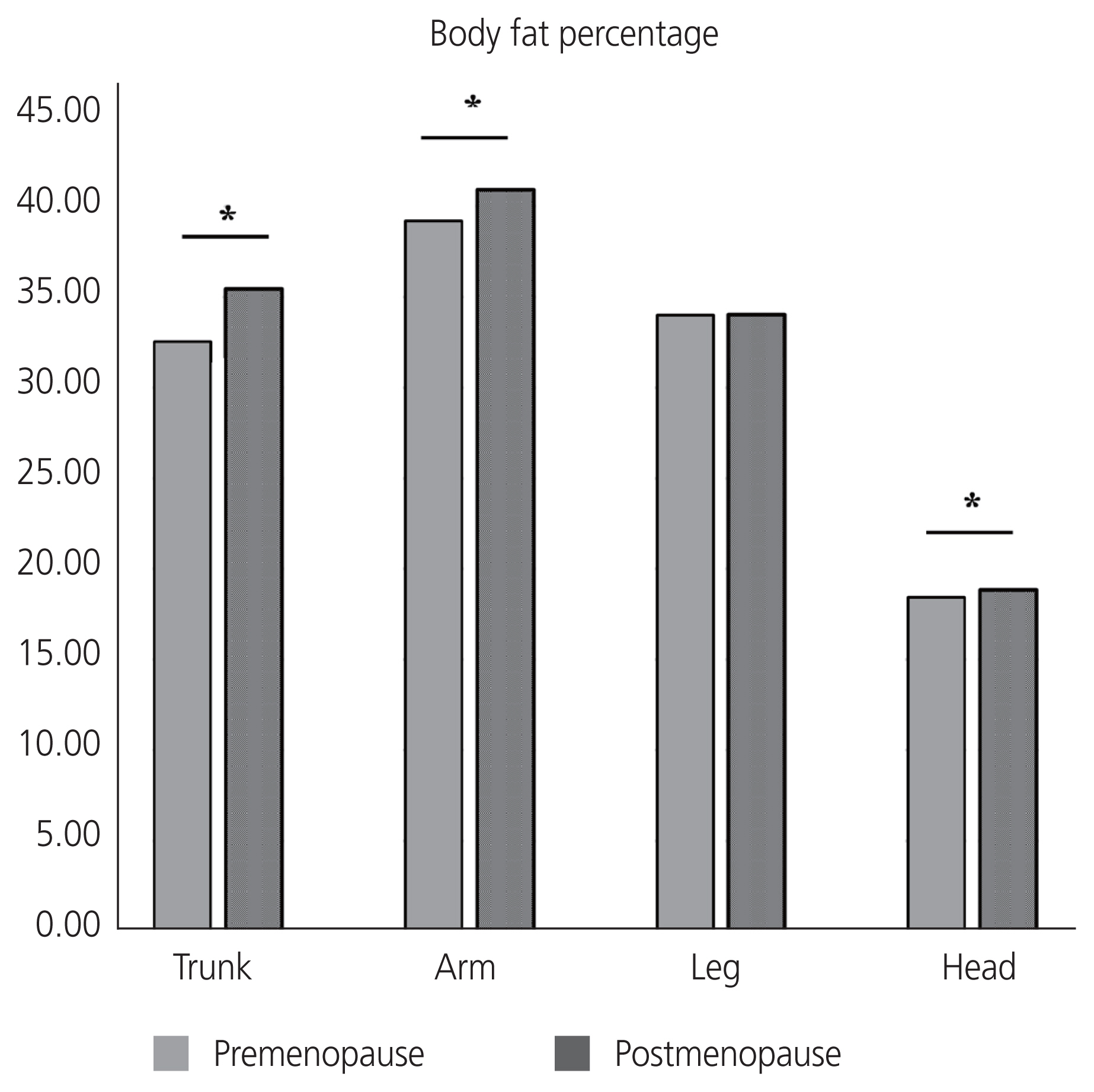
Comparison of body fat percentages between premenopausal and postmenopausal middle-aged Korean women. *P-value <0.05.
The results of multivariable regression analysis for HOMA-IR are presented in Table 2. Trunk fat percentage (estimate, 0.02; P=0.003) showed a significant positive correlation with HOMA-IR, whereas the leg fat percentage (estimate, −0.03; P<0.001) showed a negative correlation after adjusting for several confounding factors. In addition, BMI, waist circumference, diabetes mellitus, and triglyceride levels were positively correlated with HOMA-IR, whereas age, intensive physical activity, and high-density lipoprotein cholesterol level were negatively correlated with HOMA-IR in this multivariable analysis. In addition, menopausal status, current menopausal hormone therapy, carbohydrate or fat uptake, household income, and education levels were not associated with HOMA-IR (Table 2). In the subgroup analysis according to menopausal status, the leg fat percentage showed a significant negative correlation with HOMA-IR in both pre- and postmenopausal women (estimate, −0.03; P<0.001 and estimate, −0.03; P=0.001, respectively), whereas the trunk fat percentage showed a positive correlation only in premenopausal women (estimate, 0.02; P=0.010; Table 3).
Discussion
Using a nationally representative, population-based survey, this study showed that body fat distribution differed according to menopausal status among middle-aged women and was also significantly associated with insulin resistance after adjusting for several confounding factors. Trunk fat percentage and leg fat percentage were inversely associated with insulin resistance, as measured using HOMA-IR. In particular, leg fat negatively correlated with HOMA-IR regardless of menopausal status, whereas trunk fat was only associated with HOMA-IR in premenopausal women. Predicting and managing the metabolic health of middle-aged women should consider analyzing body fat distributions that show different patterns depending on menopause, rather than only using anthropometric measurements such as BMI in health checkups for those populations.
Various methodologies have been used to assess total body fat quantity or distribution, including BMI, waist circumference, DEXA, CT, and magnetic resonance imaging (MRI), in studies on women’s cardiometabolic health. Previous cross-sectional studies using DEXA showed that postmenopausal women had a greater proportion of central fat than premenopausal women [8,16]. Another study using single-slice CT also showed an increase in visceral abdominal fat with menopause [17]. In this study, we measured body fat distribution using DEXA, which is a useful technique that can accurately determine discrete fat mass cross-sectionally. It is more accurate than BMI and waist circumference to assess body composition, is less expensive and less invasive than MRI, and exposes patients to smaller doses of radiation than CT. Furthermore, DEXA is frequently performed for osteoporosis screening among middle-aged women in South Korea; therefore, body fat distribution can be easily and accurately measured along with bone mineral density in those populations. Although anthropometric measurements can be used to predict insulin resistance among middle-aged women, body fat distribution can also be measured freely using DEXA in those populations and provide valuable information regarding their metabolic health, including insulin resistance. However, further studies are needed to validate how our findings can be used to manage the health of middle-aged women.
Central adiposity has been associated with cardiovascular risk and results in adverse metabolic consequences such as dysglycemia, dyslipidemia, hypertension, and cardiovascular disease [14]. In line with these findings, this study also showed that trunk fat percentage had an independent adverse effect on insulin resistance, whereas leg fat percentage had a protective effect. The protective properties of leg fat against cardiometabolic disease risk have been suggested in several studies. Studies on adipose tissue physiology in vitro and in vivo suggest distinct properties of the leg fat depot with regard to lipolysis and fatty acid uptake [18]. It appears to be more passive than the abdominal depot and exerts its protective properties by long-term fatty acid storage, which affects insulin resistance [18]. Similarly, a larger hip circumference was associated with a lower prevalence of diabetes mellitus and dyslipidemia in a previous large population-based study [19]. Isolated adipocytes from upper-body obese women respond less to insulin activity, inhibiting lipolysis in the adipose tissue when compared with those of lower-body obesity [20]. These findings indicate that changes in body fat distribution are associated with insulin resistance and could be a marker of cardiometabolic disease risk.
To the best of our knowledge, this is the first large population-based study using a nationwide database to reveal a significant association between insulin resistance and body fat distribution according to menopausal status in middle-aged Korean women. However, this study has several limitations. First, this was a cross-sectional study that did not allow the investigation of causality. Determining the causal relationship between insulin resistance and menopause is difficult. Second, although many confounding factors were assessed and statistically controlled for, the possibility of residual confounding cannot be ruled out. Further studies are required to confirm the relationship between insulin resistance and menopause. Finally, menopausal status was not classified in detail and was determined based only on self-reported questionnaires. Further longitudinal studies with detailed definitions of menopausal status and hormonal assessments are needed.
Menopausal women have not only higher body weight, but also a different fat distribution, compared with premenopausal women. Body fat distribution was independently correlated with insulin resistance in middle-aged women. Although BMI has been used to evaluate the metabolic health of middle-aged women, our findings indicate that body fat distribution should also be assessed along with general obesity. Further studies are warranted to investigate the role of DEXA as a screening tool to predict high-risk groups for cardiometabolic diseases among middle-aged women.
Acknowledgments
This research was supported by the Statistical Support Project for Writing Medical Papers using the KNHANES, Korea University Anam Hospital.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported. Ki Hoon Ahn and Geum-Joon Cho have been an Editorial Board of Obstetrics & Gynecology Science; however, they are not involved in the peer reviewer selection, evaluation, or decision process of this article. Otherwise, no other potential conflicts of interest relevant to this article were reported.
Ethical approval
The KNHANES was approved by the Ethics Committee of the Korea Centers for Disease Control and Prevention (2007-02CON-04-P, 2008-04EXP-01-C, 2009-01CON-03-2C, 2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01-2C, 2013-07CON-03-4C, and 2013-12EXP-03-5C).
Patient consent
All study participants provided written informed consent to agree with the data to be used in national statistics and research.
Funding information
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1C1C1013579) and the Public Interest Medical Technology Research Project funded by the Ministry of Health and Welfare (MOHW, Korea; grant No. HI21C1560). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.



