Clinical outcomes of immunohistochemistry of the p53 Staining pattern in high-grade serous ovarian carcinoma
Article information
Abstract
Objective
To investigate the prevalence of p53 mutations and associated factors between immunohistochemistry (IHC) and p53 staining patterns among patients with high-grade serous ovarian carcinoma (HGSOC).
Methods
This study is a retrospective review. A total of 62 patients with HGSOC underwent surgery at Srinagarind Hospital between January 2016 and December 2020. Histological examination was performed based on a combination of morphology and IHC staining with p53. The p53 immunostaining pattern was interpreted as a missense mutation, nonsense mutation, or a wild-type pattern. Missense (p53 overexpression pattern) and nonsense (null expression p53 pattern) mutations were considered p53 mutations. A wild-type pattern was defined as a p53 non-mutation.
Results
p53 mutations were identified in 93.6% of the patients. Subgroup analysis of the p53 mutation group between the p53 overexpression pattern and the p53 null expression pattern in terms of clinicopathological characteristics and initial treatment was performed. Patients with the p53 overexpression pattern had significantly more omental metastases than those with the p53 null expression pattern (87.8% vs. 64.7%, P=0.042). There were no statistically significant differences in median progression-free survival (PFS) (9 vs. 10 months, P=0.813) or median overall survival (OS) (12 vs. 17 months, P=0.526) between the two groups.
Conclusion
The prevalence of p53 mutations in HGSOC patients in this study was 93.6%. Omental metastasis is a significant pathological factor in predicting overexpression p53 pattern in HGSC. However, IHC analysis of the p53 staining pattern did not affect OS or PFS among patients with HGSOC.
Introduction
High-grade serous ovarian carcinoma (HGSOC) is the most common histological subtype of type II epithelial ovarian cancer [1]. Eighty percent of HGSOCs are diagnosed at an advanced stage of the disease [2] and have poor prognosis [1]. Fortunately, HGSOCs respond more rapidly to platinum-based agents and poly and the enzyme poly adenosine diphosphate-ribose polymerase (PARP) inhibitors than other histological subtypes [3]. PARP inhibitors than other histological subtypes [3]. Therefore, early diagnosis of HGSOCs is crucial for appropriate treatment [4].
Serous tubal intraepithelial carcinoma from the fallopian tube fimbriae is a precursor lesion of HGSOC [5]. Moreover, 93% of HGSOC cases carry TP53 mutations [6]. However, there are no screening tools for the prevention of HGSOC to decrease mortality [7]. The detection of molecular alterations might be a promising test because nearly 100% of ovarian HGSCs contain TP53 mutations.
In 2014, the World Health Organization (WHO) recommended using histological criteria for the diagnosis of HG-SOC [8]. Based on these criteria, the accuracy of HGSC detection was 94% [9].
Assessment of ovarian carcinoma by immunohistochemical staining can improve the diagnostic accuracy and interobserver reproducibility of primary ovarian carcinomas. It can be distinguished from low-grade serous ovarian carcinoma and other metastatic adenocarcinomas [10]. Ovarian HGSC is usually positive and exhibits an abnormal pattern of p53 expression, with either diffuse, strong, or complete absence of staining [11].
p53 immunostaining pattern mutations were classified as missense, nonsense, or wild-type mutations. Missense (p53 overexpression pattern) and nonsense mutations (null expression p53 pattern) were considered p53 mutations. A wild-type pattern is defined as a p53 non-mutation [12]. However, there is limited evidence of differences in clinical outcomes between overexpression and null p53 immunostaining patterns in HGSOC.
This study aimed to investigate the prevalence of p53 mutations and the factors associated with immunohistochemistry (IHC) results of p53 staining patterns among patients with HGSOC.
Materials and methods
This study was a retrospective data review. Patients with HGSOC who underwent surgery at Srinagarind Hospital between January 2016 and December 2020 were recruited. Patients with incomplete information regarding clinical and initial treatment data and no surgical tissue pathology results were excluded. Pathological tissue was received by gynecologic oncologists at the Department of Obstetrics and Gynecology, Srinagarind Hospital, Khon Kaen University, for an initial examination before fixation in 10% neutral-buffered formalin. After fixation for 12–24 hours, the specimens were thoroughly examined macroscopically and sectioned by a gynecologic pathologist (P.K.) at the Department of Obstetrics and Gynecology, Srinagarind Hospital, Khon Kaen University. After processing using an automatic tissue processor, the tissue sections were embedded in paraffin blocks. Four-micrometer-thick slices were cut from each formalin-fixed paraffin-embedded tissue block using a rotary microtome and stained with hematoxylin and eosin. After staining, the slices were covered with a glass coverslip and sent to a board-certified pathologist specializing in gynecological oncology (P.K.). They examined all available hematoxylin and eosin-stained slides using light microscopy and made definitive pathological diagnoses.
HGSOC was diagnosed based on morphological criteria using the WHO classification 2014 [8]. It is characterized by solid, papillary, glandular, and cribriform architecture with slit-like spaces, necrosis, high-grade nuclei with bizarre forms, prominent nucleoli, abundant mitotic figures, and psammoma bodies [13]. In addition, a representative slide compatible with the criteria for each case was chosen for immunohistochemical staining.
Four-micrometer-thick slices obtained from paraffin-embedded tissue blocks were placed on superfrost plus slides. The slices were deparaffinized using a Dako PT link. Immunohistochemical staining was performed using automatic immunostaining instruments (Dako), according to the manufacturer’s recommendations. Antigen retrieval was performed using the Cell Conditional Solution. Slices were incubated with a primary antibody against paired p53 (clone DO-7: isotype: IgG2b, kappa). The p53 immunostaining pattern was interpreted as a missense mutation, nonsense mutation, or a wild-type pattern. Missense (p53 overexpression pattern) and nonsense TP53 mutations (null expression p53 pattern) were considered TP53 mutations. A wild-type pattern was defined as TP53 non-mutation or normal.
Missense TP53 mutations (p53 overexpression pattern) typically correlate with positive staining due to mutant protein accumulation owing to a loss of capacity for degradation by the proteasome [13]. Missense TP53 mutations were defined as diffuse and strong (>60% of tumor cell nuclei).
The nonsense TP53 mutation (null expression p53 pattern) was the resultant truncated form of the protein and might not be detectable by the antibody. This was defined as complete absence (0%). A wild-type pattern (TP53 non-mutation or normal) was defined as focal and weakly positive staining [13]. Histological examination was based on the combination of morphology and IHC staining of p53 by a pathologic gynecologist (P.K.) who performed a part of the original pathological diagnosis without clinical information.
Clinical information was collected from the patients’ medical records and classified using the 2014 International Federation of Gynecology and Obstetrics (FIGO) criteria [14]. Data for the remaining residual tumors were obtained from operative records. Progression-free survival (PFS) was defined as the period from the day of primary surgery to the day of recurrence or progression of the disease. Overall survival (OS) was defined as the period from the day of primary surgery to the day of death or the last confirmation of their existence. Statistical analyses were performed using STATA/SE version 10.0. The chi-square test, Fisher’s exact test, and Mann-Whitney U test were used to evaluate differences in clinicopathological factors. PFS and OS curves were generated using the Kaplan-Meier method. The log-rank test was used to compare the survival distributions. Statistical significance was defined as a P-value <0.05. This study was approved by the Khon Kaen University Ethics Committee for Human Research, Thailand, based on the Declaration of Helsinki and ICH Good Clinical Practice Guidelines (approval number: HE631413). This study was registered in the Thai Clinical Trials Registry (TCTR20210916004) on September 16, 2021.
Results
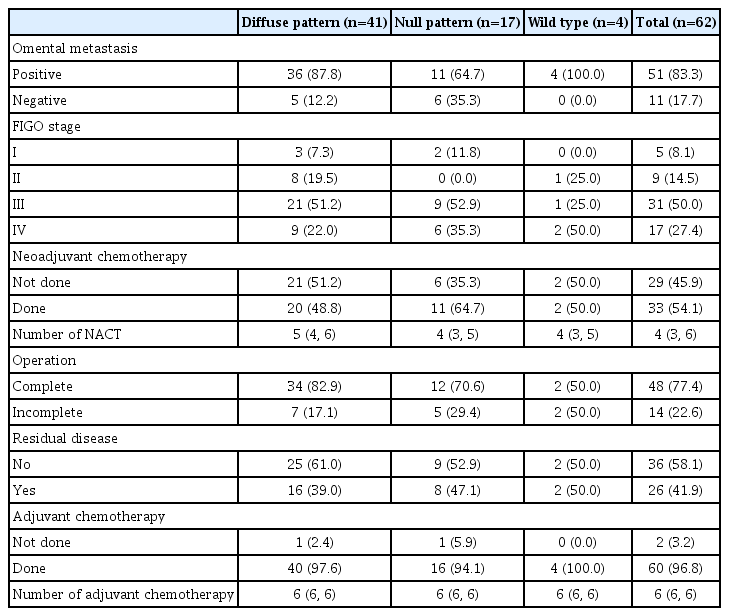
Sixty-two cases of the ovary HGSOC were included in the study. The p53 mutation was identified in 93.55% of the patients. Most of the patients were menopausal (72.6%). Half of the patients had comorbidities. The distribution of p53 immunostaining patterns of diffuse-type, null-type, and wide-type patterns was 66.13%, 27.42%, and 6.45%, respectively. The mean age±standard deviation of the patients was 57.2±10.3 years. Regarding clinical symptoms, abdominal discomfort (79%) was the main problem in HGSOC, followed by abdominal mass (46.8%) and weight loss (17.7%) (Table 1).
Most patients were diagnosed at an advanced stage (77.4%) and had omental metastases (83.3%). Half of the patients received approximately four cycles of neoadjuvant chemotherapy. Almost 80% of the patients underwent complete surgery. Half of the patients had no residual diseases. Almost all patients received adjuvant chemotherapy for six cycles (Table 2).
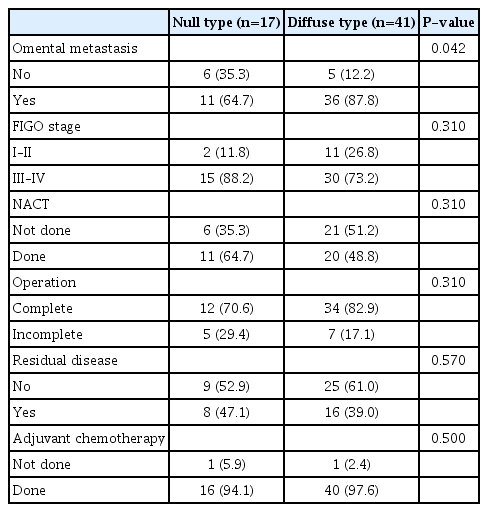
When subgroup analysis in HGSOC patients with p53 mutation was performed, omental metastasis was found to be a more statistically significant pathological factor for predicting an overexpression p53 pattern of HGSOC in the diffuse-type than in null-type staining pattern (87.8% vs. 64.7%, P=0.042). There were no statistically significant differences between the diffuse-type and null-type p53 staining patterns (Table 3). Moreover, there were no statistically significant differences in the median PFS (9 vs. 10 months, P=0.813) or median OS (12 vs. 17 months, P=0.526) between the two groups. The median follow-up period was 14 months (range, 9–28). Univariate analysis for PFS demonstrated that an advanced stage (hazard ratio [HR], 2.94; 95% confidence internva [CI], 1.14–7.57; P=0.02), incomplete surgery (HR, 2.73; 95% CI, 1.39–5.36; P=0.003), residual disease (HR, 2.84; 95% CI, 1.44–5.62; P=0.002), and adjuvant chemotherapy (HR, 0.04; 95% CI, 0.003–0.38; P<0.001) were significant prognostic factors (Table 4).
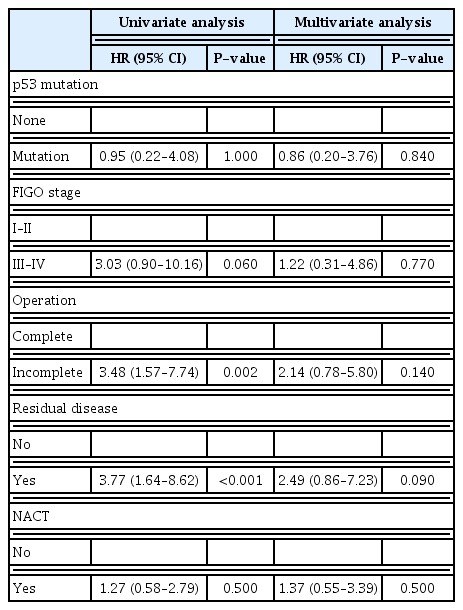
Univariate analysis demonstrated that incomplete surgery (HR, 3.48; 95% CI, 1.57–7.74; P=0.002) and residual disease (HR, 3.77; 95% CI, 1.64–8.62; P<0.001) were significant prognostic factors (Table 5). Multivariate analysis showed that only adjuvant chemotherapy was a significant prognostic factor for PFS (HR, 0.03; 95% CI, 0.003–0.38; P=0.005) and no associated factor was a significant prognostic factor for OS (Tables 4, 5).
Discussion
HGSOC is the most common subtype of epithelial ovarian malignancies. It was noted that 93% of HGSOC cases were associated with TP53 mutation [15]. Therefore, genetic testing is essential for women with a strong family history of breast cancer. However, there are currently insufficient screening tools for detecting ovarian cancer [16]. The identification of TP53 mutations found in almost all ovarian HGSCs supports the detection of this molecular alteration and may be a promising screening test. Several criteria have been established to identify individuals and families at risk of germline TP53 mutations [17]. Nevertheless, TP53 mutations were also detected in families who did not meet these criteria due to age at diagnosis, another tumor spectrum, or sporadic cancer [18]. Moreover, genetic testing is a novel strategy to detect gene mutations, but it is unavailable in some institutes. Using p53 immunohistochemical staining, TP53 mutations may be identified as diffuse-type or null-type patterns. We found that the prevalence of TP53 mutations in high-grade serous carcinoma of the ovary was 93.55% using the p53 immunohistochemical staining pattern. Diffuse and null-type staining patterns were 66.13% and 27.42%, respectively.
Kuhn et al. [19] reported that the distribution of diffuse-type (missense) and null-type (nonsense) p53 immunohistochemical staining patterns in HGSOC was 60% and 39%, respectively. Moreover, Na et al. [20] and Yemelyanova et al. [10] reported that the mutation pattern of p53 immunostaining was found in 94.4–96.75% of HGSOC patients, based on the combination of p53 overexpression (>60%) and complete lack of p53 expression (0%). These results are comparable to those of the present study. However, Cole et al. [21], using different cut-off values (>70% for overexpression and <5% for lack of expression), noted matching results between immunostaining and mutational analyses in 95.8% of HGSOC cases. In our study, the mutation pattern of p53 immunostaining was found in 93.54% of cases when cutoff values of >60% for overexpression and 0% for lack of expression were used.
The diagnosis of HGSOC was based on histologic morphology according to the 2014 WHO classification. However, these criteria may misclassify HGSOC by approximately 6%, particularly in TP53 non-mutation, especially the histological subtypes of endometrioid and low-grade serous carcinoma [9]. Thus, careful attention to histologic features and the selected use of immunohistochemical stains can avoid misclassification as HGSOC. In our study, we found that the wild-type pattern (TP53 non-mutation) in four patients (6.45%) may be misclassified as HGSOC, as mentioned in a previous study; however, after a review by gynecologic pathologists, the final diagnosis was HGSOC.
Matulonis et al. [22] noted that patients with HGSOC typically present with gastrointestinal symptoms, including abdominal pain, bloating, nausea, constipation, and anorexia, showing similar results to our study. Gastrointestinal symptoms include abdominal discomfort, abdominal mass, and weight loss.
Mutant TP53 is associated with cancer metastasis, particularly in adipocyte-rich environments. Hu et al. [23] reported that mutant TP53 in HGSOC cells interacts with sterol regulatory element-binding proteins and guanidinoacetate N-methyltransferase, leading to increased gene expression of fatty acids (FAs) and cholesterol biosynthesis, and the inhibition of FA oxidation (FAO). Consequently, increased lipid anabolism promotes tumor growth and progression. Once omental metastasis is detected, mutant TP53, together with adipocyte-derived interleukin-8, upregulates FA-binding protein 4 expression, accelerating tumor growth in adipocyterich metastatic environments. This suggests that mutant TP53 might play a crucial role in HGSOC progression.
According to a previous review [24], most HGSOCs are usually diagnosed at an advanced stage and have a poor prognosis, similar to our study. Nieman et al. [25] showed that 80% of patients with HGSOC presented with omental metastasis, similar to our study, which demonstrated that 83.3% of patients with HGSOC had omental metastasis. In addition, we found that omental metastasis was a significant pathological factor for predicting the p53 overexpression pattern in HGSOC (87.8% vs. 64.7%, P=0.042).
Our study reported that TP53 mutation was not a significant prognostic factor for PFS or OS. Günakan et al. [26] reported p53 gene expression in patients with stage 1a epithelial ovarian cancer (EOC) was not associated with OS or disease-free survival in the short term, which was similar to our study. However, we found that advanced stage (HR, 2.94; 95% CI, 1.14–7.57; P=0.02), incomplete surgery (HR, 2.73; 95% CI, 1.39–5.36; P=0.003), residual disease (HR, 2.84; 95% CI, 1.44–5.62; P=0.002), and adjuvant chemotherapy (HR, 0.04; 95% CI, 0.003–0.38; P<0.001) were significant prognostic factors in univariate analysis for PFS. However, after multivariate analysis, only adjuvant chemotherapy was found to be a significant prognostic factor for PFS (HR, 0.03; 95% CI, 0.003–0.38; P=0.005).
Univariate analysis of OS demonstrated that incomplete surgery (HR, 3.48; 95% CI, 1.57–7.74; P=0.002) and residual disease (HR, 3.77; 95% CI, 1.64–8.62; P<0.001) were significant prognostic factors. However, after multivariate analysis, no associated factor was found to be a significant prognostic factor for OS. Horowitz et al. [27] found that patients who underwent optimal cytoreductive surgery with no residual tumor had more promising outcomes than those who underwent suboptimal surgery. HGSOC is an epithelial ovarian cancer in which the removal of metastatic tumors has been found to improve OS [28]. However, our study did not show significant results from the multivariable analysis because of the small sample size. We reported the clinical outcomes of patients with HGSOC using the p53 immunohistochemical staining pattern, which has recently been limited in the available evidence, making this the strength of our study.
This study was a retrospective review conducted at a single institution. Moreover, the diagnosis of HGSOC in all participants was based on the combination of morphology and IHC staining with p53 by one pathologic gynecologist in our institute who performed part of the original pathological diagnoses without clinical information; thus, there are limitations. Further multicenter and prospective studies should be conducted to confirm the clinical significance of HGSOC and to increase the effect size of treatment outcomes in the future. However, although there are some limitations, we believe that our results have clinical implications for using IHC of the p53 staining pattern to predict clinical outcomes, especially omental metastasis, among patients with HGSOC.
The prevalence of TP53 mutations in HGSOC in this study was 93%. Omental metastasis is a significant pathological factor in predicting overexpression p53 patterns in HGSC. However, IHC analysis of the p53 staining pattersn did not affect OS or PFS among patients with HGSC.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
This study was approved by the Khon Kaen University Ethics Committee for Human Research, Thailand, based on the Declaration of Helsinki and ICH Good Clinical Practice Guidelines (approval number HE631413). This study was registered in the Thai Clinical Trials Registry (TCTR20210916004) on September 16, 2021.
Patient consent
Informed consent was waived due to the retrospective and anonymous nature of the study and participants.
Funding information
This study was supported by the Khon Kaen University (IN63363).