Management of inoperable endometrial cancer
Article information
Abstract
Some endometrial cancer (EMC) patients are not good candidates for primary surgery. The three major types of treatment for inoperable EMC are radiation therapy, chemotherapy, or their combination as neoadjuvant treatment before surgery. Radiation therapy alone (of different modes) has been used as the sole definitive therapeutic modality, particularly for early-stage disease that is limited to the uterine body and cervix with or without parametrial invasion. The most common treatment modality is neoadjuvant treatment before surgery. Postoperative adjuvant treatment is also occasionally used, depending mainly on the sites of the disease and the results of surgery. Data on neoadjuvant hormonal or radiation therapy are limited, with studies focusing on laboratory outcomes or having only a small number of patients. Most neoadjuvant treatments before surgery involved chemotherapy and fewer combined chemoradiotherapy. Surgery was generally performed, particularly in patients who had shown responses or at least stable disease to neoadjuvant treatment. Perioperative outcomes after neoadjuvant treatment were superior to those after primary surgery, whereas survival data were still inconsistent. Features that had or tended to have a favorable prognosis were younger age, early-stage disease, response to neoadjuvant treatment, low preoperative cancer antigen-125 level, and optimal surgery. Among different modalities of neoadjuvant treatment, which has become a frequent mode of treatment, neoadjuvant chemotherapy was more common than radiation therapy alone or chemoradiation.
Introduction
Surgical treatment with total hysterectomy and bilateral salpingo-oophorectomy is the standard primary treatment for endometrial cancer (EMC). Unless the patients show some risk features necessitating adjuvant treatment, surgery may be the only treatment they will receive.
The prognosis of patients with EMC is generally good because most patients have early-stage disease at presentation. However, approximately 10–15% of EMC patients present with advanced-stage cancer [1]. This latter group of patients is unlikely to undergo safe or successful surgical removal; therefore, they are not good candidates for primary surgery. Moreover, the general condition of some patients at the time of diagnosis may not be optimal for performing major surgery. The combined incidence of these two conditions is also not an uncommon phenomenon. Therefore, surgery is not an appropriate primary treatment option for such patients. In these two scenarios, neoadjuvant therapy before definitive surgical treatment is likely to be a better option. Another relevant scenario involves the presence of severe comorbid illnesses that are frequently found in elderly and frail EMC patients. This latter group of patients may not be able to undergo definite surgical treatment at any time, especially when their life expectancy may be already shortened by medical illnesses themselves.
For cases showing advanced disease and an equivocal possibility of optimal cytoreduction on physical examination, several imaging studies, such as computerized tomography (CT) scans, magnetic resonance imaging (MRI), and positron emission tomography scans are currently available to help surgeons evaluate the extent of disease prior to surgery [2–5]. This approach is especially helpful for high-grade or serous carcinomas, which frequently show a higher probability of extrauterine or distant metastasis than other types of EMC [2,6].
Most of the existing information regarding the treatment of inoperable EMC is derived from retrospective studies or case series. Without evidence-based data from randomized studies, combined data from the available reports may help gynecologic oncologists to select an alternative treatment option for EMC patients who are not candidates for primary surgery. This study collected and summarized data from a literature review of relevant studies based on the treatment intention (definitive or neoadjuvant). Treatment administered with a palliative aim, either chemotherapy, hormonal therapy, or best supportive care, is beyond the scope of this review.
Methods
We searched the MEDLINE database for studies and reports published from January 1997 to December 2020. We searched for all studies that reported treatments for EMC patients who did not receive surgery as the primary treatment due to advanced disease or medical illnesses. Only studies with curative intent (definitive treatment) were included. Studies that provided palliative treatment were excluded.
Results
A review of the literature revealed that most of the studies on this topic were retrospective studies without randomized trials. The three major types of treatment used for inoperable EMC were radiation therapy, chemotherapy, combinations of radiation therapy and chemotherapy, and interval surgery. The findings for these types of treatment are described below.
1. Radiation as a definitive therapy
Radiation has long been used as a definitive therapy, particularly for EMC patients with comorbid illnesses that preclude surgery and those with disease that is limited to the pelvis. The different modes of radiation therapy that have been used are external pelvic radiation therapy (EPRT), brachytherapy (intracavitary radiation therapy [ICRT]), or their combination.
One study suggested aggressive (multimodal) radiation therapy even for early-stage EMC [7]. This study described the findings for 101 EMC patients aged 39–94 years who could not undergo surgery due to medical problems [7]. All patients had clinical stage I disease, with the majority (82%) showing cancer invading less than half of the myometrium, while the remaining patients showed no myometrial invasion. The modes of radiation used were EPRT plus split-field and ICRT (61%), ICRT alone (26%), EPRT plus ICRT (10%), and EPRT alone (3%). The 5-year disease-free survival (DFS) and overall survival (OS) rates were >80%. The survival rates of patients who were older than 75 years were lower than those of younger patients: DFS, 55% vs. 84%; OS, 78% vs. 84%.
Other studies have reported inconsistent data regarding the benefits of adding ICRT to EPRT [8,9]. One study used the National Cancer Institute’s Surveillance, Epidemiology, and End Results public database to analyze the prognostic factors and outcomes in 997 stage I–II EMC patients who did not undergo surgical treatment [8]. Among these patients, 39% received radiation therapy as EPRT alone (21%) or ICRT with or without EPRT (18%). The type of radiation therapy was not associated with OS or EMC-specific survival. On the other hand, EPRT alone was sufficient, since the additional use of ICRT did not improve survival in comparison with EPRT alone [8]. However, in another small study involving 11 EMC patients with stage I–III disease who could not undergo ICRT after EPRT and image-guided stereotactic body radiation [9], nearly half (45%) of the patients experienced locoregional disease progression at a median follow-up of 10 months. Progression-free survival (PFS) at 18 months was only 41%. Stage IB disease was much worse than stage IA disease, with a PFS of 33% in comparison with 100%.
The type of ICRT used appeared to have no major influence on the outcome. One study reported successful treatment of 45 clinical low-risk inoperable EMC patients (stage I, small grade 1–2 tumors <2 cm, with less than 50% myometrial invasion) with the use of only high-dose rate image-guided tandem and cylinder ICRT as a definitive treatment [10]. Vaginal bleeding was controlled in 97% of patients. Clinical complete radiologic response from MRI and 2-year locoregional control were achieved in 90% of the patients, with cancer-specific survival and OS rates of 86% and 97%, respectively [10]. Another study also reported a high success rate of treatment with low-dose rate ICRT using Heyman’s capsules in 44 cases of medically inoperable clinical stage I EMC [11], with 5-year disease-specific survival and OS rates of 88% and 61%, respectively. The authors concluded that low dose rate ICRT yielded outcomes similar to those of surgery and high dose rate ICRT.
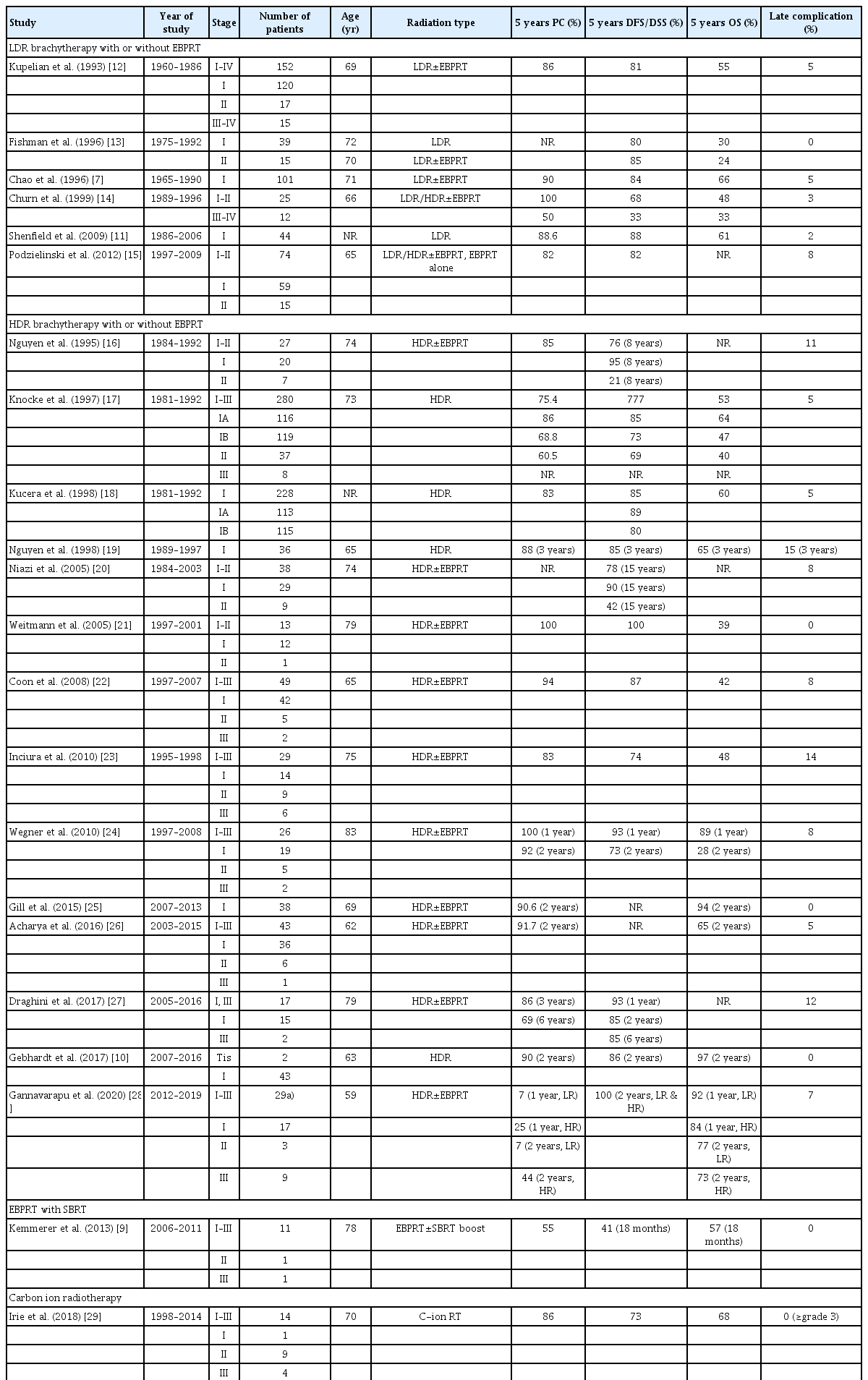
Data from the studies that used radiation therapy as a definitive treatment for EMC are shown in Table 1 [7,9–29]. Aside from the disease stage, the detailed characteristics of EMC may facilitate tailoring of the type of radiation therapy. The American Brachytherapy Society has supported the use of MRI and 3D planning for definitive radiation treatment of medically inoperable EMC [30]. The gross tumor volume, depth of myometrial invasion, clinical target volume, and organs at risk can be assessed with this approach. For clinical stage I tumors without deep myometrial invasion and lymph node involvement, ICRT alone can be used. On the other hand, additional EPRT may be considered when MRI is not available to assess these features. In brief, the society summarized that ICRT is sufficient for stage I disease with tumor grade 1–2, and no deep myometrial invasion on MRI. External pelvic radiation along with ICRT is recommended in stage I disease with deep myometrial invasion or no MRI assessment (or only CT scan), and in stage II–III EMC that is still limited within the pelvis [30].
The American Brachytherapy Society also reported survival outcomes according to the type of radiation therapy from studies over different periods of time [30]. In summary, the 5-year cancer-specific survival of patients with stage I EMC treated with only ICRT (either low-or high-dose) or with additional EPRT ranged from 65–85% and up to 95%, respectively. The survival rates with only ICRT or with additional EPRT were, respectively, 40–69% and 88% for stage II disease and 14% and 57% for stage III disease. The differences in the ranges of survival rates obtained with different modes of radiation therapy across various studies may be partly due to unavailable or inconsistent use of MRI to assess tumor features (especially in clinical stage I EMC) and thereby tailor the type of radiation in each study, especially in studies conducted in the remote past.
2. Neoadjuvant treatment prior to surgery
In the modern era, with rapid advancements in medical technology and medications (chemotherapy), most gynecologic oncologists may opt to use neoadjuvant treatment prior to definitive surgery. Neoadjuvant treatment is usually aimed at reducing the bulk or extent of the tumor, making surgery possible. On the other hand, EMC patients with some medical illnesses may need medical care to improve their condition before major surgery.
For physicians (mostly gynecologic oncologists), the selection of an appropriate treatment, which is usually a choice between definitive treatment or only palliative management, for patients with inoperable EMC is rather difficult. In such cases, the potential survival benefit has to be weighed against potential adverse reactions and treatment futility. Such evaluations should take several factors into consideration, including the patient’s general health status, extent of disease, chance of undergoing surgery, surgical outcomes, and clinicopathological features of EMC itself.
Histologic type and grade are important prognostic factors for EMC [31–33]. Tumor histopathology and grade have been shown to be associated with the response to chemotherapy in the neoadjuvant setting [34,35]. One study found that grade 2 endometrioid tumors had a significantly better response to chemotherapy than grade 3 endometrioid tumors (72% vs. 43%, respectively) [35]. Surprisingly, the response rate for grade 1 tumors was only 46%. These findings require confirmation in additional studies because only 16% of the cases were in the primary setting (adjuvant or neoadjuvant therapy) and 84% involved chemotherapy in the recurrent setting, when tumor grade and biological behavior may be different from those in primary tumors. Another study found associations among pathological features, chemotherapy response score, and survival [34].
Aside from low-grade and less-aggressive histopathology, the ability to undergo surgical treatment appears to be an important favorable prognostic factor for EMC patients who receive neoadjuvant treatment of any type. One study reviewed the data of 59 patients with unresectable stage II to IVA EMC who underwent curative-intent treatment with neoadjuvant radiation (more than half also received concurrent chemotherapy) (47%), neoadjuvant chemotherapy (NACT) (29%), or definitive radiation (24%) [36]. The overall surgery rate was 66%:82% in the NACT group and 79% in the neoadjuvant radiation group. Overall, 19% of the patients experienced at least grade 2 gastrointestinal, genitourinary, vaginal, or musculoskeletal toxicities. The survival benefit of surgery was also demonstrated. The 3-year survival rates of patients who could undergo surgery (66%) were significantly higher than those who were not able to undergo surgery: 84% vs. 41% for OS and 56% vs. 11% for DFS, respectively.
A positive effect of surgical treatment on survival was also found in studies that focused only on chemotherapy as a neoadjuvant treatment. One study found that patients who could undergo interval debulking surgery (IDS) had superior OS than other patients: 15–16 months compared to 5–6 months [37]. Some features that were reported to be associated with a higher chance of surgical treatment were younger age, earlier stage, or endometrioid histopathology [36].
The surgical outcome is even more important than the ability to undergo surgery. Although the benefit of optimal cytoreduction in EMC is not as well recognized as in ovarian cancer, few studies have identified optimal cytoreduction as a favorable prognostic factor for EMC patients. Rajkumar et al. [38] described optimal cytoreduction (<1 cm) as an independent good prognostic factor for survival in 45 patients with stage IIIC/IV EC patients who received neoadjuvant therapy (38% received chemotherapy). Patients who had suboptimal cytoreduction showed a 3.55-fold increased risk of death, with significantly shorter survival than those who received optimal surgery: 12 months vs. 16 months for PFS and 18 months vs. 29 months for OS. Another study in 30 serous stage IV (transperitoneal spread) patients, which was the only prospective study to date, reported successful surgery (80% optimal cytoreduction and 92% without residual tumor) in 28 patients who showed some response after NACT (complete, partial response, or stable disease). The authors also reported a PFS of 13 months and OS of 23 months [39]. Predictive factors for successful surgery are useful considering their important influence on surgical outcomes. Some clinical features associated with a higher chance of surgical treatment, such as young age, lower stage, endometrioid histopathology, or response to neoadjuvant therapy, have also been reported to be associated with better surgical outcomes [36,37]. A reduction in preoperative cancer antigen (CA)-125 levels is another factor. One study in 115 patients with advanced-stage Müllerian carcinoma who had NACT demonstrated that a CA-125 reduction of at least 90% was a favorable prognostic feature associated with complete IDS, fewer bowel resections, and absence of viable tumor or presence of only microscopic disease [40].
These previously described predictive factors for good surgical outcomes (younger age, endometrioid histology) as well as low-grade tumors were also good prognostic features for survival [7,36]. Other favorable factors were preoperative CA 125 level <20 mIU/mL (HR 0.37 for survival) [40], absence of tumor infiltration, presence of tumor necrosis [39], and high chemotherapy response score [34]. On the other hand, unfavorable prognostic factors that have been reported to date include poor performance status and the presence of bowel disease [38].
Although the Cancer Genome Atlas Research Network has categorized EMC on the basis of distinct molecular and genetic features to guide appropriate therapy for individual patients [41], this classification was not widely practiced, especially in the remote past and in settings with limited resources. Hence, data on individualized therapy, especially in neoadjuvant settings for inoperable EMC, are not currently available.
A physician may consider all currently available prognostic factors, counsel the patient, and make appropriate decisions regarding the type of neoadjuvant treatment. Data on hormonal therapy, radiation, chemotherapy, or their combinations as neoadjuvant treatment prior to surgery are provided below.
1) Neoadjuvant hormonal therapy
Since EMC is a hormone-related cancer, many studies have reported the use of hormonal therapy for advanced or recurrent EMC [42], medical unfit early-stage EMC [43] or in adjuvant settings [44]. However, only a few studies have assessed the role of hormonal agents in neoadjuvant treatment [45,46]. One study compared the effects of letrozole, anastrozole, and exemestane in 38 patients [45]. The findings showed an improvement in clinical signs (decreased endometrial thickness) and symptoms (relief of abdominal pain and uterine discharge), most frequently with anastrozole. Laboratory investigations revealed reductions in intratumoral aromatase and blood estradiol levels more frequently after anastrozole and letrozole administration, whereas tumor progesterone receptor levels were markedly lower after exemestane administration [45]. A subsequent report from the same group of authors described superior effects of 2–4 weeks of aromatase inhibitor treatment in comparison with 2–9 weeks of metformin treatment in terms of endometrial thickness, grade change, and proliferative index among 38 EMC patients (it was unclear whether the patients were the same as those in the authors’ preceding report) [47]. Another small trial randomized 24 EMC patients with any histopathology to receive anastrozole (n=16) or placebo (n=8) for at least 14 days prior to surgery [46]. Expression of estrogen receptor alpha, androgen receptor, and Ki-67 (but not Bcl2 and progesterone receptor) was significantly lower after treatment with anastrozole.
Advances in knowledge from clinical studies on neoadjuvant hormonal therapy for EMC are unlikely, probably because the cytostatic effect of hormonal agents to slow tumor progression is not the aim in a neoadjuvant setting. In these settings, the induction of a cytotoxic effect to reduce the tumor load through the more remarkable effects of chemotherapy or radiotherapy in reducing tumor bulk is probably preferred.
2) Neoadjuvant radiation therapy
Few studies have reported the use of radiation as a neoadjuvant treatment in locally advanced EMC, wherein the disease has spread beyond the uterine body to the cervix or parametrium. In these studies, the mode of radiation was either EPRT combined with ICRT or ICRT alone.
Some studies have used preoperative ICRT alone for EMC that was limited to the uterus or cervix (stage I or II) [48–50]. One small study included 12 patients who received high-dose ICRT for clinical stage I–II [50]. In that study, surgery revealed a complete pathological response in two patients and only microscopic residual disease in the other five patients, and the 2-year disease-free and cause-specific survival rates were 88% and 100%, respectively. Two other non-English reports also described success after preoperative ICRT in EMC [48,49]. One study from Poland showed that 51% of the 35 patients who underwent surgery had no residual cancer after ICRT [48]. Another study from France described the findings for 780 clinical stage I–II EMC patients who received preoperative low-dose ICRT and underwent long-term follow-up of over 10 years [49]. Postoperatively, pelvic radiation was used if necessary. The 5-year local control rate in that study was 93%, with an 86% DFS and 84% OS.
Other studies have used combinations of EPRT and ICRT for the treatment of EMC with cervical or parametrial extension. One study by Vargo et al. [51] reported the use of neoadjuvant EPRT (predominantly intensity-modulated radiation therapy) and image-based high-dose rate ICRT with or without chemotherapy, followed by surgery in 36 patients with EMC with or without parametrium involvement. Extrafascial hysterectomy performed at a median of 6 weeks after radiotherapy revealed a clinical complete response in 91% of cervical lesions, whereas pathological complete response was achieved in 24% of patients. The 3-year DFS and OS rates were 73% and 100%, respectively. Another study by the same group of authors focused on the safety of extrafascial hysterectomy either by laparotomy or laparoscopy after neoadjuvant external beam radiotherapy followed by image-based high-dose rate ICRT in 29 EMC patients [52]. Except for vaginal complications, which occurred frequently (33%) and were particularly prominent in patients who underwent laparoscopic surgery, the incidence of other perioperative complications was low in both laparotomy and laparoscopic approaches (3–6%). The authors also noted no significant correlation between radiotherapy dose and postoperative complication rates.
3) Neoadjuvant chemotherapy
The role of NACT followed by delayed primary surgery, which is more commonly called IDS, in ovarian cancer has been well-recognized and commonly practiced [53,54]. This is because ovarian cancer commonly presents with advanced-stage disease, for which primary surgery is not suitable. Unlike ovarian cancer, most patients with EMC usually present with early-stage disease, leading to limited evidence-based data regarding NACT in EMC.
Nevertheless, several case reports [55,56], retrospective studies [57–60], and one prospective study [61] describing the use of NACT before IDS in advanced-stage EMC have been identified. In the mid-2000s, Despierre et al. [55] and Takami et al. [56] reported the findings for three EMC patients with advanced-stage clear cell or serous carcinoma who showed complete or remarkable response (disappearance of lung metastasis) after three cycles of NACT. The patient underwent successful surgery without residual disease.
Generally, IDS is performed in patients who show some responses (preferably complete or partial response rather than stable disease) after a few cycles of NACT. Response to chemotherapy is a key factor determining whether patients can undergo IDS. Aside from the efficacy of NACT, another important factor influencing the response is probably the aggressiveness of the EMC itself (as reflected by the histopathological findings). This was demonstrated in several studies [37,59]. One small study by Khouri et al. [37] included 39 EMC patients showing unresectable tumors with aggressive histology of clear cells, carcinosarcoma, or neuroendocrine carcinomas aside from endometrioid carcinoma (only 28%). After NACT (85% of the patients received paclitaxel and carboplatin), 59% of the patients could not undergo IDS because of disease progression (70%) or persistent unresectable disease (17%). Another recent study collected data for 102 advanced-stage EMCs of all histologic types [59]. Outcomes after NACT (89% of the patients received paclitaxel and carboplatin) differed by histopathological subgroup. The response rate to NACT is 64% in endometrioid carcinoma and 80% in serous carcinoma [59].
The surgical or perioperative outcomes of IDS appear to be better than those of primary surgery. Two retrospective studies compared the treatment outcomes of stage IV serous EMC patients who underwent NACT followed by IDS or primary surgery: 15 patients in each group using a propensity-matched algorithm in one study [60] and 10 patients (NACT) or 34 patients (primary surgery) in another study using a simple cohort design [58,60]. Better operative and post-operative outcomes were demonstrated in patients who underwent NACT and IDS than in those who underwent primary surgery followed by chemotherapy, in terms of shorter operative time, lower transfusion rate, and a shorter hospital stay [58,60]. Although not statistically significant, the rate of no gross residual disease was 2-fold higher in the NACT/IDS (70%) group than in the primary (32%) group (same figures in both studies) [58,60].
Studies that compared the survival of EMC patients who had undergone NACT/IDS after primary surgery/chemotherapy yielded inconsistent findings. Among these, two retrospective studies that reported improved operative and postoperative outcomes as described earlier demonstrated comparable survival between the patients who underwent NACT or primary surgery: PFS, 10–12 months vs. 12–15 months; OC, 17 months vs. 18–21 months [58,60]. However, other studies found lower survival rates in patients who underwent NACT/ICT than in those who had primary surgery/ chemotherapy [57,61]. One large multicenter study from Japan reviewed the data of 426 patients with clinical or surgical stage IVB EMC [57]. The median OS was the longest after primary surgery (279 patients; 21 months), followed by primary chemotherapy (NACT) (125 patients; 12 months), and the lowest with only palliative care (125 patients; 1 month). The survival difference between patients who underwent primary surgery or primary chemotherapy in this study must be interpreted with caution because patients in the primary surgery group had more favorable prognostic features (better performance status and fewer extra-abdominal metastases). Among a subset of patients who responded to NACT and were able to undergo surgery, the OS was similar to that in patients who underwent primary surgery.
Another study retrieved data from the National Cancer Database to assess the influence of primary treatment on the survival of over 48,000 patients with stage III/IV EMC between 2004 and 2015 [61]. The majority of the patients underwent primary surgery followed by adjuvant chemotherapy (83%), whereas the others received only chemotherapy (12%) or NACT/IDS (5%). The OS was the highest among patients who underwent surgery and adjuvant chemotherapy, followed by those who received NACT before surgery, and was the worst among those who received chemotherapy alone: 61 months vs. 25 months vs. 11 months, respectively. In comparison with chemotherapy alone, the hazard ratios were 0.32 for primary surgery and adjuvant chemotherapy and 0.44 for NACT followed by IDS. Subgroup analyses by stage (stage III or stage IV) or histology (type I or type II EMC) showed a significant OS benefit in patients who underwent primary surgery in comparison with the other two groups, regardless of stage or histology. Nevertheless, NACT showed a significantly superior survival benefit over chemotherapy alone, except for cases with type I stage III cancer, which showed no survival difference. One observation from this study was that although the study included a large number of patients, the percentage of patients who had NACT/IDS was small (5%). Furthermore, data on the prognostic features of patients in each group were not available for analysis. Hence, a definite conclusion is unlikely.
Although there are no solid evidence-based data from randomized controlled trials for the use of NACT in EMC, findings from several reports were consistent, showing more favorable surgical outcomes. Although survival data of NACT/IDS compared to primary surgery/chemotherapy are still inconsistent, NACT appears to be a common treatment option for inoperable EMC in recent years.
We were aware of the activity of immunotherapeutic agents (anti-programmed death-1 monoclonal antibody) either alone [62] or in combination with tyrosine kinase inhibitors in advanced EMC that had failed prior to systemic therapy [63]. The demonstrated efficacy of these agents in phase I/II studies led to phase III trials that had recently reported superior survival outcomes over standard chemotherapy with paclitaxel and carboplatin [64] or are still ongoing [65]. Whether these agents will be used in the future as neoadjuvant treatment in advanced and inoperable EMC is yet to be seen.
4) Neoadjuvant chemoradiation therapy
The use of combined chemoradiation, especially in a concurrent pattern, is well recognized as a standard treatment for locally advanced-stage cervical cancer. However, this is not the situation in EMC, since the activity of concurrent chemoradiation in cases of EMC has been tested as an adjuvant therapy after surgery in only a few randomized trials [66–68]. In fact, there are even fewer data regarding the use of concurrent chemoradiation for EMC in a neoadjuvant setting.
To date, only three reports on this topic have been published. One case report described a patient with stage IVB EMC who presented with a large uterine tumor with wide-spread retroperitoneal and inguinal nodal metastases that became resectable after chemotherapy (carboplatin and 5-fluorouracil) followed by radiation therapy [69]. The patient achieved a 4-year recurrence-free outcome. Combinations of chemotherapy and radiation can be tailored according to the characteristic features of the cancer. This was supported by another case report in which multimodality treatment was performed in an EMC patient with grade 3 cancer extending to the cervix, parametrium, pelvic side wall, and pelvic and para-aortic nodal metastases [70]. The patient received a course of neoadjuvant chemoradiotherapy with pelvic and para-aortic field intensity-modulated radiation therapy, in combination with low-dose weekly cisplatin. This was followed by intrauterine tandem and interstitial needles based on the initial extent of the disease, with an additional high-dose rate ICRT prior to surgery. Complete surgical staging 1 month after the radiation revealed a complete pathological response.
The third report was a retrospective study of 34 patients with type II EMC (serous, carcinosarcoma, and clear cell carcinoma) of stage II–III [71] who received external pelvic radiation plus ICRT concurrent with platinum chemotherapy (mainly cisplatin) as neoadjuvant treatment. Over 90% of cancer cases were downstaged. The surgical approach was laparoscopic (41%), laparotomic (38%), or robotic (21%). Free surgical margins were achieved in 94% of patients. Pathologic complete response was demonstrated in 15% of the patients, while 35% showed only microscopic residual disease. While 6% of the patients required blood transfusion, no intraoperative complications were encountered. Postoperative complications were found in 39% of the patients: readmission or ileus (12% each), wound infection (9%), and pulmonary emboli or sepsis (3% each). The 2-year local, regional, and distant control rates were approximately 80%, with 53% DFS and 64% OS rates. The results of this study should be interpreted with caution because they were not simply due to the effect of concurrent chemoradiation treatment. Neoadjuvant or adjuvant treatment was also administered to 12% and 79% of the patients, respectively. Notably, 12% of patients received NACT before chemoradiation and surgery followed by adjuvant chemotherapy.
Although not directly relevant to the topic of this review, one more study merits a mention in the present investigation. This study used a national registry of over 20,000 stage III EMC patients diagnosed between 2004 and 2013 to assess the patterns-of-care and OS benefits of chemoradiation versus chemotherapy or radiation alone as adjuvant therapy [72]. The use of chemoradiation increased significantly during the study period. A significantly decreased risk of death (hazard ratio, 0.62) was associated with the use of treatment combinations over a single mode of treatment. However, the trend for combined chemoradiation as neoadjuvant therapy for primary inoperable EMC remains unclear.
One major caveat is the tolerability of EMC patients who are old and frequently have comorbid illnesses. Some patients may not be able to endure the adverse reactions of combined treatments such as neoadjuvant therapy. Furthermore, some patients may require adjuvant therapy post-operatively. Thus, neoadjuvant concurrent chemoradiation should not be performed until more studies can demonstrate its clear benefit.
Discussion
Radiation therapy alone can be administered as definitive treatment for early-stage primary inoperable EMC. MRI should be used to tailor the mode of radiation therapy (according to the extent of tumor) when it is administered as the sole treatment. Neoadjuvant treatment with radiation, chemotherapy, or a combination of these prior to surgery can be used for all cases of early- or advanced-stage disease. The most common neoadjuvant treatment is chemotherapy, whereas less data is available regarding radiation or chemoradiation. Neoadjuvant treatment yielded better perioperative outcomes, but the survival rates associated with neoadjuvant treatment were inconsistent in comparison with those obtained with primary surgery.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
This study does not require approval of the Institutional Review Board because no patient data is contained in this article. The study was performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
Written informed consent and the use of images from patients are not required for the publication.
Author contributions
SP: Data curation, writing original draft, review, and editing; SC: Data curation, writing original draft; ST: Conceptualization, data curation, supervision, writing original draft, review, and editing. All authors have read and approved the final manuscript.
Funding information
None.
Data availability
The authors declare that data supporting the findings of this study are available in the article.