A model for predicting gestational diabetes mellitus in early pregnancy: a prospective study in Thailand
Article information
Abstract
Objective
To develop a predictive model using the risk factors of gestational diabetes mellitus (GDM) and construct a predictive nomogram for GDM risk in women during early pregnancy.
Methods
A prospective study was conducted in two tertiary hospitals among pregnant women with gestational age ≤14 weeks. Early GDM was diagnosed if an abnormal 100 g oral glucose tolerance test was detected using the Carpenter and Coustan criteria after an abnormal 50 g glucose challenge test. The factors included in the model were ACOG risk factors; maternal age; family history of hypertensive disorder in pregnancy; family history of dyslipidemia; gravida; parity; histories of preterm birth, early fetal death, abortion, stillbirth, and low birth weight; and glycated hemoglobin (HbA1c) levels. The predictive models for early GDM were analyzed using multiple logistic regression analyses. The nomograms were constructed, and their discrimination ability and predictive accuracy were tested.
Results
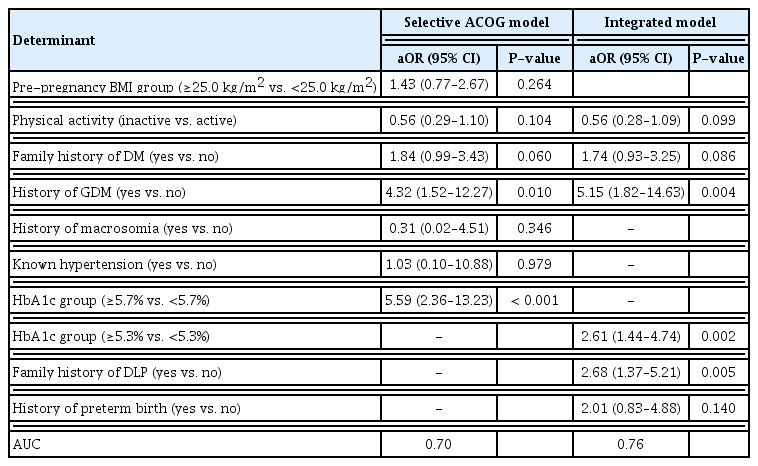
Of the 553 pregnant women, 54 (9.8%) were diagnosed with early GDM. In the integrated model, there was a history of GDM (adjusted odds ratio [aOR], 5.15; 95% confidence interval [CI], 1.82–14.63; P=0.004), HbA1c threshold ≥5.3% (aOR, 2.61; 95% CI, 1.44–4.74; P=0.002), and family history of dyslipidemia (aOR, 2.68; 95% CI, 1.37–5.21; P=0.005). The integrated nomogram model showed that a history of GDM had a high impact on the risk of early GDM. Its discrimination and mean absolute error were 0.76 and 0.009, respectively.
Conclusion
Application of the predictive model and nomogram will help healthcare providers investigate the probability of early GDM, especially in resource-limited countries.
Introduction
Gestational diabetes mellitus (GDM), described as glucose intolerance with first recognition during pregnancy, is one of the most common disorders found in pregnant women [1,2]. Although GDM is treatable, maternal and perinatal complications have been widely reported [3,4]. Screening and diagnosis of GDM is commonly performed at 24–28 weeks of gestation via universal screening or other strategies [5]. A systematic review that analyzed results from 11 studies in seven countries and examined the burden of GDM reported that the combined prevalence of GDM screened in the second or third trimester was 10.1% [6]. However, another systematic review reported that the prevalence of GDM in early pregnancy varied from 0.8% to 22.9%. This raises the concern of undiagnosed type 2 diabetes mellitus (T2DM) before pregnancy [7,8]
Early screening is the key to assure early detection and effective treatment, resulting in lower consequences for both the mother and the baby [3]. However, there is currently a lack of consensus regarding the screening for GDM in early pregnancy. The United States Preventive Services Task Force Recommendation Statement recommended insufficient evidence to support the benefit or harm of screening GDM before 24 weeks in all pregnant women [9]. Moreover, universal screening and laboratory tests result in a great burden on healthcare budgets, out-of-pocket payments in the context of no health insurance, and poor infrastructure, particularly in low- and middle-income countries [10]. Therefore, a predictive model for screening for the risk of GDM at the first antenatal visit for early detection of GDM is needed.
Glycated hemoglobin (HbA1c) levels have been used to monitor and diagnose diabetes in non-pregnant individuals due to its ability to indicate average plasma glucose over a previous three-month period [1,11]. HbA1c level has been proposed as a potential indicator for GDM screening or to ascertain undiagnosed T2DM in early pregnancy. However, cut-off thresholds have not been determined because they depend on the study population, and they are not part of routine antenatal care [4,12].
Previous studies have proposed risk-scoring systems or models using their risk factors for predicting GDM [13–15], and the American College of Obstetricians and Gynecologists (ACOG) 2018 recently updated the risk factors for women at risk of pre-gestational diabetes or early GDM [4]. The idea of integrating the risk factors from the ACOG with the risk-scoring concept seems to be challenging for the implementation of a predictive model in resource-limited countries. Therefore, this study aimed to create a predictive model using the risk factors of GDM and construct a nomogram to predict the probability of GDM risk in women during early pregnancy.
Materials and methods
A prospective study was conducted in two tertiary hospitals: Songklanagarind Hospital, a university hospital, and Naradhiwas Rajanagarindra Hospital, a tertiary hospital, in Southern Thailand. All pregnant Thai women with gestational age of ≤14 weeks, who attended antenatal care at these study hospitals during March 14, 2018 and March 13, 2020, were included. Those who had known medical conditions (such as diabetes mellitus, anemia, or mental illness) and incomplete test results were excluded from the analysis. The sample size was calculated based on the estimated sensitivity of the risk model at 87% [13], with an acceptable error of 10%, a 95% confidence interval (CI), and a 9% expected prevalence of early GDM [16]. At least 484 pregnant women were screened to achieve an estimated 44 women with early GDM.
The main outcome measure of this study was the prediction of early GDM, defined as diagnosis of GDM at a gestational age of ≤14 weeks via a universal 2-step approach using a 50 g glucose challenge test (GCT) followed by a 100 g oral glucose tolerance test (OGTT) [4]. Women who had a positive 50 g GCT (1-hour plasma glucose level ≥140 mg/dL) underwent a diagnostic 100 g OGTT after an overnight fast. GDM was diagnosed when at least two of four plasma glucose values of the 100 g OGTT met the Carpenter and Coustan criteria as follows: fasting plasma glucose ≥95 mg/dL, 1-hour plasma glucose ≥180 mg/dL, 2-hour plasma glucose ≥155 mg/dL, and 3-hours plasma glucose ≥140 mg/dL [17].
The independent variables used to construct the predictive model included maternal age, family history of hypertensive disorder in pregnancy (HDP), family history of dyslipidemia (DLP), gravida, parity, history of preterm birth, history of early fetal death (<20 weeks), history of abortion, history of stillbirth (fetal death ≥20 weeks and neonatal death), or history of low birth weight. Additionally, selective ACOG risk factors for early GDM included pre-pregnancy body mass index (BMI), physical activity, family history of diabetes mellitus (DM), history of GDM, history of macrosomia, hypertension, and HbA1c levels. Pre-pregnancy BMI was calculated by dividing the pre-pregnancy weight (kg) by the height squared (m2) and categorized into BMI <25 versus ≥25 kg/m2. Physical activity, involving activity at work, travel to and from places, and recreational activities in the previous week, were measured using the Global Physical Activity Guidelines [18,19]. A family history of DM, HDP, and DLP in this study were defined as a history of diseases in the mother, father, and/or sibling.
All pregnant women who visited the antenatal care unit at the study hospitals and met the inclusion and exclusion criteria were recruited. After obtaining written consent, face-to-face interviews were conducted to obtain their personal information and obstetric history. Blood samples were collected to determine 1-hour plasma glucose levels after 50 g GCT and HbA1c levels. All blood samples were kept in cold storage before being delivered to the clinical chemistry laboratory unit (Songklanagarind Hospital, Prince of Songkla University, Songkhla, Thailand). Glucose levels were measured using a Cobas 8,000 modular analyzer series (Roche Diagnostics GmbH, Mannheim, Germany) using the hexokinase method. HbA1c levels (%) were determined using a Capillarys 3 Tera (Sebia, Lisses, France) via the capillary electrophoresis technique.
Data were entered in Epidata version 3.1 (The EpiData Association, Odense, Denmark, 2004) and analyzed using the R statistical software version 3.6.3 (The R Foundation for Statistical Computing, Vienna, Austria, 2020). Differences in descriptive information between groups were analyzed using the chi-square test or Fisher’s exact test for categorical variables, and an independent t-test for continuous variables. The ACOG uses a HbA1c cut-off threshold of 5.7% (39 mmol/mL); however, the optimal HbA1c threshold was calculated in this study using the “OptimalCutpoints” package, regarding the maximum product of specificity and sensitivity method [20].
The integrated model using the risk factors from the ACOG and additional risk factors was determined by multiple logistic regression analysis. Factors associated with early GDM, with a P-value <0.2 in univariate analysis, were initially used for the first model of multiple logistic regression analysis. A backward-stepwise method was then used to keep the final model with significant factors (P-value <0.05). The best receiver operating characteristic curve was considered to select the factors for placement into the predictive models for early GDM, in addition to their significance. The nomogram and calibration curve of the prediction were constructed using the regression modeling strategies (rms) in the “rms” package, which includes the functions for regression models. A bootstrapping approach with 1,000 resamples was performed to internally validate the nomogram to achieve a degree of optimism with the model [21,22]. The calibration curve and the Harrell concordance index (C-index) were applied to determine its predictive accuracy and discriminatory ability. A C-index >0.5 indicated a high prediction ability.
Results
A total of 585 pregnant women attending their first prenatal visit were included in the study. The median gestational age was eight weeks (interquartile range, 6.0–10.0). Thirty-two were excluded from the analysis due to the lack of 100 g OGTT (n=30) and HbA1c (n=2) results. Of the 553 women analyzed, early GDM was found in 54 women (9.8%). Of 244 women who underwent 100 g OGTT, fasting plasma glucose ranged from 61 to 120 mg/dL. The flow diagram of the study is shown in Fig. 1. The personal information and obstetric history of women without GDM and early GDM are presented in Table 1. Maternal age, family history of DLP, history of early fetal death, history of preterm birth, and history of low birth weight were significantly different between the groups.
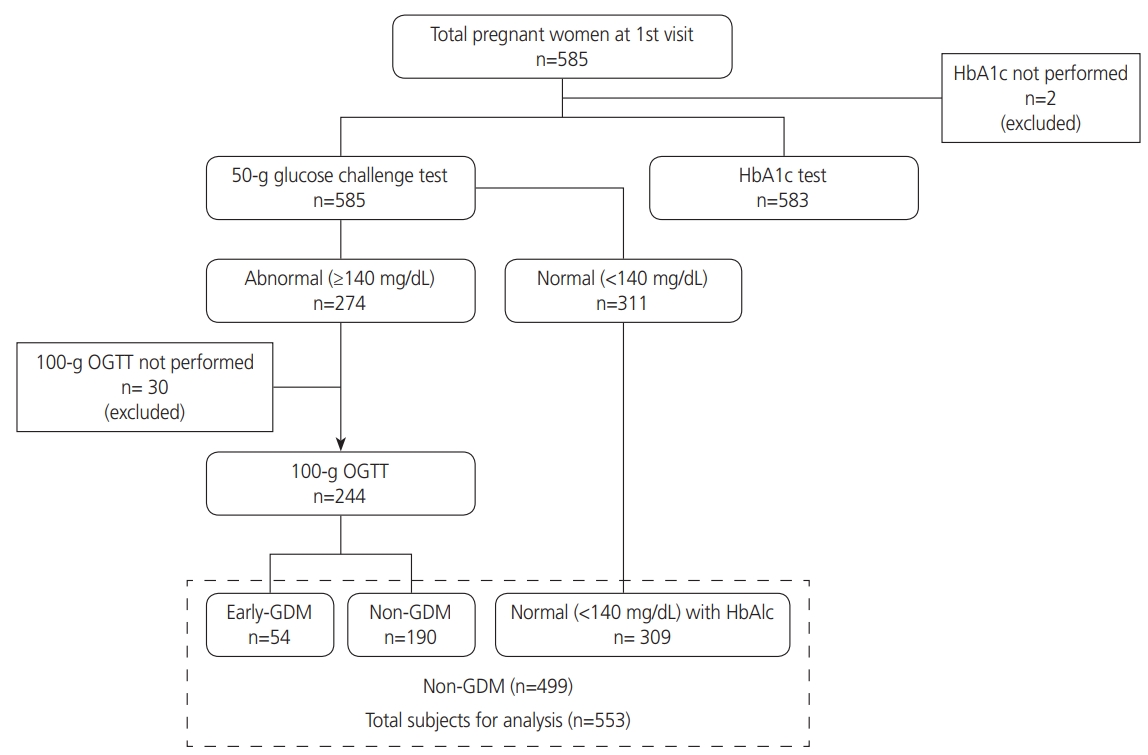
Flow chart of the study. HbA1c, glycated hemoglobin; OGTT, oral glucose tolerance test; GDM, gestational diabetes mellitus.
Risk factors for early GDM, selected from the ACOG recommendations between women with and without early GDM are shown in Table 2. Pregnant women with early GDM were more likely to have a pre-pregnancy BMI ≥25 kg/m2, low inactive physical activity, family history of DM, history of GDM, and HbA1c level over the cut-off threshold compared to those without GDM. The optimal cut-point analysis presented 5.3% as the best cut-off threshold for HbA1c, showing the area under the curve (AUC) at 0.67 (95% CI, 0.58–0.75). Of the 54 women with early GDM, 24.1% and 55.6% had an HbA1c threshold of ≥5.7% and ≥5.3%, respectively.
The final models of risk factors associated with early GDM for the selective ACOG and integrated models are presented in Table 3. In the selective ACOG model, the odds of detecting early GDM significantly increased in women with a history of GDM (adjusted odds ratio [aOR], 4.32; 95% CI, 1.52–12.27; P=0.010) and HbA1c threshold ≥5.7% (aOR, 5.59; 95% CI, 2.36–13.23; P<0.001). In the integrated model, history of GDM (aOR, 5.15; 95% CI, 1.82–14.63; P=0.004), HbA1c threshold ≥5.3% (aOR, 2.61; 95% CI, 1.44–4.74; P=0.002), and family history of DLP (aOR, 2.68; 95% CI, 1.37–5.21, P=0.005) significantly increased the odds of having early GDM.
The factors for the integrated model showing the best AUC prediction of early GDM were history of GDM, HbA1c threshold 35.3%, family history of DLP, family history of DM, physical inactivity, and history of preterm birth, as shown in the nomogram constructed (Fig. 2A). A history of GDM was found to have the highest impact on the prediction of early GDM. The discrimination of the nomogram yielded a c-index of 0.76 (95% CI, 0.75–0.76). The mean absolute error of the calibration curve of the nomogram was 0.009 (Fig. 2B).
Discussion
GDM in early pregnancy was found in 1 of 10 pregnant women in Southern Thailand. The history of GDM and HbA1c levels significantly remained in the model using the selective ACOG factors for predicting early GDM, whereas history of GDM, HbA1c levels, and family history of DLP did so in the integrated model. A visual nomogram, constructed using family and obstetric history, physical activity and HbA1c in the integrated model, is applicable to increase the probability of early GDM detection during GDM screening in routine practice.
A personal and family history of GDM were found to be predictors for early GDM in this study; these results concur with those of a retrospective study in China, which was a machine learning study using the variables extracted in early pregnancy within 12 weeks of gestation [23]; however, information on gestational age, when GDM was diagnosed, was not provided [23]. This finding was of concern as it might indicate the problem of unrecognized DM in women with a history of GDM [1]. Based on several professional recommendations, women with GDM should be followed up to have a 75 g glucose tolerance test performed at least 6–12 weeks after delivery to diagnose glucose intolerance or DM [24,25]. However, women with GDM were less likely to undergo postpartum testing, as recommended [26].
HbA1c level was another significant risk factor in both the selective and integrated models for predicting early GDM. This finding was consistent with the results of Arbib et al. [15] in which HbA1c level was considered one factor to add to the predictive model to screen for GDM in early pregnancy. Although HbA1c level was a strong predictor, it was not recommended for use as an individual factor to predict GDM [27,28] or as an alternative test to diagnose GDM because of its variation in cut-off and diagnostic performance [12,29]. A family history of DLP appeared to be a significant factor associated with early GDM in the integrated model. Previous studies have also reported an association between DLP and GDM, in which they reported higher detection of dyslipidemia in women with GDM than in those without GDM [30,31]. However, there is no clear evidence to explain the effect of a family history of DLP on GDM.
From the literature search, five studies that presented nomograms for GDM were found. These included three studies predicting the risk of GDM [32–34], one study predicting insulin requirement [35], and another study predicting postpartum T2DM [36]. In these three previous studies for predicting the risk of GDM, they used 75 g OGTT as their diagnostic criteria, not 100 g OGTT, as in our study. Additionally, these three studies did not predict GDM during early pregnancy. Although the nomograms were constructed by selecting all significant and non-significant factors from ACOG recommendations [4], the discriminative performance of the nomograms for predicting GDM at early pregnancy was quite similar to the findings of previous studies conducted in China and South Africa for predicting GDM at 24–28 weeks of pregnancy [33,34]. From a systematic review of diagnostic test accuracy studies in Southeast Asia, the prevalence of GDM screened using a two-step approach at 24–28 weeks ranged from 7.1% to 28.6% in Thailand [37]. Two retrospective studies were published in 2020 [38] and 2021 [16], which showed the rates of early onset GDM at 18.9% and 9.2%, respectively. The variation in the reported early GDM rate depends on universal screening or screening in high-risk pregnant women.
This study reassures the need for assessment of glucose intolerance and DM during the postpartum period for all women with GDM [39]. Additionally, it highlights the alarming and unrecognized prevalence of DM or early GDM in low-income and middle-income countries. The findings in this study should be considered in the context of the following limitations: first, the nomogram must be tested for its validation due to its first development. Second, prior-pregnancy factors might have influenced the higher risk scores. Third, personal information included family history that might have caused under-or over-estimation due to recall bias. Fourth, generalizability may be limited in other populations with different ethnicities or risk of diseases. Finally, the effect of GDM treatment in early pregnancy is required to be further explored as a previous study reported that the use of different diagnostic criteria influenced the incidence of macrosomia differently [40].
In conclusion, the nomogram is an easy-to-use and low-cost tool to predict the probability of early onset GDM at the first antenatal visit. Therefore, it could facilitate healthcare providers in distinguishing those who are either high- or low-risk for GDM in early pregnancy. The health outcomes on the benefits and harms of GDM screening in early pregnancy should be further studied.
Acknowledgments
We thank the directors of the study hospitals for their permission to conduct the study, and the health personnel at antenatal care clinics for their support during data collection. We also acknowledge Mr. Andrew Jonathan Tait, Office of International Affairs, Faculty of Medicine, Prince of Songkla University for his help with proofreading.
Notes
Conflict of interest
All authors report no conflicts of interest.
Ethical approval
This study was approved by the Ethics Committees of the Faculty of Medicine, Prince of Songkla University, Thailand, in accordance with the Declaration of Helsinki (REC number: 60-413-18-1). All eligible pregnant women signed a consent form before participating in the study. All data were collected using codes to maintain an anonymous process and were kept confidentially.
Patient consent
All recruited pregnant women were informed and signed the consent form before participating in this study. No use of images from participants was involved, thus the consent for image use was not required for publication.
Funding information
This research was financially supported by the Thailand Science Research and Innovation (TSRI) Research Career Development Grant (RSA) (Grant Number RSA6180009) and the Targeted Research Grants Program of the Faculty of Medicine, Prince of Songkla University, Thailand. The authors declare no conflict of interest.