Venous thromboembolism associated with combined oral contraceptive use: a single-institution experience
Article information
Abstract
Objective
Combined oral contraceptives (COCs) are used for various reasons. However, venous thromboembolism (VTE), a significant side effect, can be fatal. This study reports the first case series in Korea involving patients with COC-associated VTE registered at a university hospital.
Methods
This study recruited 13 patients diagnosed with COC-associated VTE between June 2006 and May 2018. Risk factors, including age, body mass index, smoking habits, estrogen dosage, type of progestin, and duration of COC use, were evaluated.
Results
Among patients with VTE, 9 showed pulmonary embolism (PE) concomitant with deep vein thrombosis (DVT). However, the remaining patients showed DVT (1 patient), PE (1 patient), and cerebral venous thrombosis (2 patients). The median duration between the onset of symptoms and a hospital visit was 3 days, and it sometimes took as long as 32 days. Among the 10 patients with PE, 1 high-risk group and 2 intermediate-high risk groups were treated with tissue plasminogen activators before anticoagulants. There were no cases of recurrence among patients who continued to take anticoagulants for 3 months.
Conclusion
These findings emphasize that healthcare professionals who prescribe or dispense COCs to women must inform them of the risk of VTE, including the risk factors, differences in risk depending on the type of progestin present in the product, and pertinent signs and symptoms. Efforts should also be made to inform patients of VTE, even through information campaigns such as brochures. Most importantly, women should remain alert for signs and symptoms of VTE when using COCs.
Introduction
Since their introduction in the 1960s, combined oral contraceptives (COCs) have been used worldwide as safe, effective, and reversible protection against pregnancy. COCs are used by 8% of women of reproductive age worldwide and constitute the most common contraception method in industrialized countries. It has been reported that among women of reproductive age, the percentages of those who take contraceptives are as follows: 17.8% in Europe and North America, 21.9% in Australia and New Zealand, 5.2% in East and Southeast Asia, and 3.3% in South Korea [1]. In addition to their contraceptive action, noncontraceptive use of COCs includes treating disorders such as abnormal uterine bleeding, dysmenorrhea, and polycystic ovarian syndrome [2,3]. However, concerns over cardiovascular side effects have led to modified COC compositions to reduce the risk of venous thromboembolism (VTE) and other adverse cardiovascular events [4].
VTE is an unavoidable adverse event associated with COCs, and the risk is higher in COC users (3–9 per 10,000 people/ yr) than in non-users (1–5 per 10,000 people/yr) [5]. The risk appears to be higher in preparations containing high doses of estrogen or newer progestins, including desogestrel, gestodene, and drospirenone [6–8]. Said risk can be explained by increased levels of prothrombin and factor VII, and decreased levels of factor V being more pronounced when using 3rd- or 4th-generation COCs [9]. COC-associated VTE is a very rare condition in Korean women, which has caused it to be overlooked by numerous physicians and healthcare professionals. A few case reports have described COC-induced VTE or arterial thrombosis in Korean women [10–14]. However, these describe a single case across different hospitals (Table 1), not as a single or multicenter case series. This study reports the first case series involving 13 patients with COC-associated VTE registered at a thrombosis center at a university hospital.
Materials and methods
The records of 13 patients with COC-associated venous thromboembolic events registered at a thrombosis center at a Soonchunhyang University Hospital between June 2006 and May 2018 were reviewed. Laboratory tests were performed for thrombophilia, including fibrinogen and D-dimer, and for the estimation of proteins C and S and antiphospholipid antibodies.
VTE was diagnosed based on the patient’s clinical presentation and confirmed using vascular imaging, including computed tomography or magnetic resonance imaging. DVT was diagnosed when there was no compressibility of the veins of the lower extremities or direct visualization of a clot. PE was diagnosed when there was a filling defect in the pulmonary artery or its branches. Cerebral venous thrombosis was diagnosed when the blood clot was present in the dural venous sinuses, cortical vein, or deep cerebral vein.
Risk factors related to COC-associated VTE, including age, body mass index (BMI), smoking habits, estrogen dosage, type of progestin, and duration of COC use, were evaluated. Additionally, COC use, clinical diagnosis, treatment, and hospital discharge outcomes were also recorded. The study protocol was reviewed and approved by Soonchunhyang University Hospital’s Institutional Review Board and ethics committee (IRB No. 2018-04-011-001).
Results
A total of 13 patients were recruited between 2006 and 2018, 9 of whom were hospitalized for COC-related VTE. The mean length of hospitalization was 5.9 days (1–14 days).
1. Baseline characteristics
The patients’ mean age was 33.4±10.1 years. For the patients’ age distribution, 5 were 20–29 years old, 3 were 30–39 years old, 4 were 40–49 years old, and 1 was over 50 years old (Table 2). The mean BMI was 23.3±4.0 kg/m2. Three patients were overweight (BMI 23.0–24.9 kg/m2), and 4 were obese (BMI ≥25.0 kg/m2). Three patients were smokers at the time of the VTE event, and 10 patients denied a history of smoking. The median duration between the onset of symptoms and the hospital visit was 3 days (range, 0–32 days).
2. Indications and duration of the COC administration
Dysmenorrhea was the most common indication for COC administration (5 cases), followed by abnormal uterine bleeding (3 cases), contraception (2 cases), polycystic ovarian syndrome (1 case), acne (1 case), and postoperative treatment of leiomyoma (1 case). The median COC administration duration was 6 months (0.5120 months) (Table 2).
3. Constituents of COCs
In 2 patients, the constituents of the COCs were not recorded, and the estrogen dosage and the type of progestin could not be confirmed. Of the 11 remaining patients, 7 identified the COC they took, which contained 20 μg of ethinyl estradiol (EE), while the remaining 4 took COCs containing 30 μg of EE. For the COC progestin component, 1 patient took a 2nd-generation COC containing levonorgestrel, 1 took a 3rd-generation COC containing desogestrel, 3 took a 3rd-generation COC containing gestodene, and 6 took a 4th-generation COC containing drospirenone. Table 2 shows how long each patient used the COCs before their VTE diagnoses.
4. Anatomical site of VTE
The most common thromboembolic event associated with COC use is pulmonary embolism (PE) concomitant with deep vein thrombosis (DVT). Nine patients displayed PE concomitant with DVT, 1 had isolated DVT, 1 had isolated PE, and 2 had cerebral venous thrombosis (CVT; Table 2). Of the 10 DVT cases, 9 affected the deep veins in the lower extremities and 1 affected the deep veins in the upper extremity.
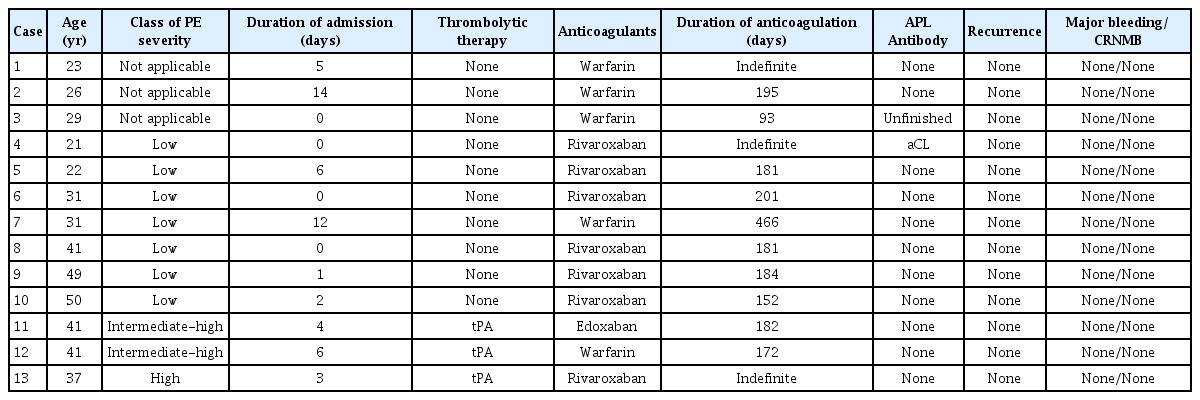
5. Class of PE severity and therapeutic strategies
Ten patients with PE were classified as low-, intermediate-, or high-risk according to the simplified PE severity index (sPESI). One patient was classified as having a high-risk of hypotension. Two patients were classified as intermediate-high-risk with right ventricular hypokinesia and positive cardiac biomarkers, and the remaining patients were classified as low-risk. High-risk and intermediate-high-risk patients immediately received a tissue plasminogen activator (tPA) followed by anticoagulants, while low-risk patients received only anticoagulants (Fig. 1). Table 3 outlines the patients’ severity classifications and the anticoagulants they received (i.e., edoxaban, rivaroxaban, or warfarin).
6. Duration of anticoagulation and outcome of treatment
Anticoagulation was continued for more than 3 months in all patients. In this study, 3 patients consistently took anticoagulants. Two of the 3 patients received indefinite treatment for combined antiphospholipid Ab syndrome, and the remaining patient who experienced shock during a VTE event received medication indefinitely due to fear of re-experiencing shocks.
7. Recurrence of bleeding after treatment
None of the patients experienced a recurrence of VTE or a major or clinically relevant non-major bleeding event following the thrombolytic therapy or anticoagulation therapy.
Discussion
Using hormonal contraceptives contributes to a significant percentage of VTE occurring among women of childbearing age, and VTE is the most important determinant of the risk-benefit profile associated with hormonal contraceptives [4]. The risk of VTE is dependent on age, BMI, the presence of inherited or acquired thrombophilia, duration of COC use, type of pill, and exposure to additional risk factors [15]. Based on a Danish cohort study, incidences of VTE increase with age from 1.84 per 10,000 woman-years in women aged 15–19 to 6.59 per 10,000 woman-years in women aged 45–49 [16–18]. Meanwhile, the VTE risk in women using COCs is attributed to changes in hemostasis [19]. Estrogen increases gene expression and the plasma levels of coagulation factors and decreases anticoagulation factor levels, including proteins C and S and tissue factor pathway inhibitors [20–22]. In contrast, progestin upregulates the gene expression of protein S [23,24].
COCs are classified as prescription drugs in most developed countries, and case reports describing their adverse effects are regularly investigated and recorded. However, most COCs are approved over-the-counter drugs for easy accessibility in South Korea; hence, there is a lack of ways to report this issue. An analysis of adult Korean women taking COCs showed that they were not familiar with the exact use of oral contraceptives, their purpose of use, or their side effects. When women visited hospitals for COC treatment, many doctors gave them prescriptions without explaining the use of COCs, and the same can be said for pharmacists. Most women were taking COCs based on incorrect information from the Internet without the help of experts [25]. Although a few case reports described single cases of COC-induced VTE in different Korean hospitals, this study is the first case series to report COC-associated VTE in the country.
The results of this study indicate that 11 of 13 patients with COC-associated VTE did not use COCs for contraceptive purposes. Aside from the 2 patients who could not identify the COCs given to them, 10 of the 11 patients received a 3rd- or 4th-generation COC. The use of COCs in women diagnosed with obesity will likely increase their risk of VTE, and a BMI greater than 25 kg/m2 is an independent risk factor for thromboembolism [26–28]. However, this study showed that only 4 patients were obese. The small percentage of patients classified as such could be attributed to the small sample size.
Three patients reported a history of smoking while using COCs. The synergistic effect of smoking and COC use increases the risk of VTE, particularly in those over 35 years of age [29,30]. This result emphasizes that women of that age group with a history of smoking should be informed about the higher risk of VTE associated with COC use.
Those who were taking a short-term medication remembered the medication period without a break-in. In comparison, patients who were taking a long-term medication may not recall the exact break-in period and accurately know how long they took and when they stopped taking the COCs. If the 3 patients with missing data were excluded, 50% of VTE occurred within the first 3 months of use. This result is consistent with previous reports indicating that VTE risk is highest during the same duration [19,31,32], which suggests that long-term COC use does not increase complications.
In this case series, all but 3 patients reported using low-dose EEs (20 or 30 μg). The fact that most patients were using 3rd- or 4th-generation COCs in this study supported the finding that the risk of COC-associated VTE is 50–80% higher with COCs containing gestodene, desogestrel, and drospirenone than those containing levonorgestrel [33,34].
For the type of VTE, PE simultaneously occurring with DVT was the most common form of COC-associated VTE in this study, and given the severity of PE associated with COC use, 30% of patients with PE required thrombolytic therapy as the initial therapeutic approach. Although thrombosis in the cerebral venous system is generally rare, cerebral venous thrombosis was detected in 2 of the 13 patients in this study. Prothrombotic conditions, including antithrombin III, protein C and S deficiencies, factor V Leiden gene mutations, cancer, and oral contraceptive use, are known risk factors for CVT [35]. Two meta-analyses reported that the risk of CVT increased in COC users (relative risk: 15.9, 95% confidence interval [CI]: 6.98–36.2; odds ratio: 5.59, 95% CI: 3.95–7.91) [36,37]. None of the patients in this series demonstrated prothrombotic conditions, and COC use was the only risk factor for CVT.
In this study, anticoagulation therapy was continued for more than 3 months in all patients. Three patients consistently took anticoagulants, and none of the patients experienced VTE recurrence. According to a study on patients who experienced an initial untriggered VTE, oral contraceptives could be a provoking factor, with a significantly lower recurrence rate after treatment with anticoagulants for 5 to 7 months compared to women with unproven factors [38]. Anticoagulation medications were stopped after 6 months of treatment because the risk of recurrence in patients with unprovoked VTE with negative D-dimers is low enough to justify stopping therapy, and the rate of VTE recurrence in hormonal users is significantly lower [39,40].
The results indicated that most of the patients in this study were prescribed COCs for non-contraceptive purposes, and they delayed going to the hospital even after the onset of VTE symptoms. These findings emphasize that healthcare professionals who prescribe or dispense COCs to women must inform them of the risk of VTE, including the risk factors, differences in risk depending on the type of progestin present in the product, and pertinent signs and symptoms. Efforts should also be made to inform patients of VTE, even through information campaigns such as brochures. Most importantly, women should remain alert for signs and symptoms of VTE when using COCs.
Notes
Eun Sil Lee has been an Editorial Board of Obstetrics & Gynecology Science; however, she was not involved in the peer reviewer selection, evaluation, or decision process of this article. Otherwise, no other potential conflicts of interest relevant to this article was reported.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study protocol was reviewed and approved by Soonchunhyang University Hospital’s Institutional Review Board and ethics committee (IRB No. 2018-04-011-001). The study was performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
Written informed consent and the use of images from patients are not required for the publication.
Funding information
None.
Acknowledgements
The authors would like to thank the thrombosis center nurse, Mi-ok Hwang, who helped organize the data.