Comparison of single-port laparoscopy and laparotomy in early ovarian cancer surgical staging
Article information
Abstract
Objectives
The aims of this study were to assess the feasibility of single-port laparoscopic surgical staging (SPLS) in early ovarian cancer and to compare the surgical outcomes of SPLS with those of staging laparotomy.
Methods
Between January 2014 and December 2018, 40 patients underwent SPLS and 41 patients underwent staging laparotomy at Yonsei Cancer Center. The patients were diagnosed with International Federation of Gynecology and Obstetrics (FIGO) stage I ovarian cancer. Variables such as patient age, body mass index (BMI), tumor size, FIGO stage, and perioperative surgical outcomes and survival outcomes of SPLS and laparotomy were compared.
Results
The total operation time was similar between the 2 groups (SPLS: 201.4 vs. laparotomy: 203.0 minutes, P=0.806). The median tumor diameters in the SPLS and laparotomy groups were 11.0 (2.5–28 cm) and 15.4 (6–40 cm), respectively (P=0.001). The SPLS group had lower tumor spillage rate (5.0% vs. 19.5%, P=0.047), less intraoperative blood loss (102.0 vs. 371.5 mL, P<0.001), less postoperative pain, and shorter postoperative hospital stay (5 vs. 9.5 days, P<0.001). The intraoperative major complication rate was similar between groups (2.5% vs. 4.9%, P=0.571). There was no significant difference in progression-free survival between the 2 groups (P=0.945). There were no deaths in either group.
Conclusion
SPLS is feasible in early ovarian cancer and has better perioperative surgical outcomes, in some aspects, than staging laparotomy without compromising survival outcomes. SPLS could be performed in patients suspected to have early ovarian cancer.
Introduction
Ovarian cancer is the second most common gynecologic malignancy, with the highest mortality rate [1]. Because it is difficult to detect ovarian cancer at an early stage, it is usually not diagnosed until the advanced stage. As with most cancers, ovarian cancer has a high 5-year relative survival rate (92.6%) if detected early [2]. Ovarian cancer is surgically staged, and an optimal staging operation is required to determine the exact stage, treatment plan, and prognosis. The surgical methods for staging ovarian cancer include total hysterectomy, bilateral salpingo-oophorectomy, pelvic and paraaortic lymphadenectomy, omentectomy, peritoneal biopsy, and peritoneal washing for cytology [3,4].
Recently, many gynecologic surgeries have been performed using a minimally invasive approach compared with the previous invasive approaches, with improved surgical outcomes such as better cosmetic results, less postoperative pain and intraoperative blood loss, and shorter hospital stay. In particular, single-port laparoscopy using a single incision in the umbilicus maximizes the advantages of conventional laparoscopy [5–8].
There have been few studies on single-port laparoscopy for ovarian cancer staging [9,10]. Staging laparotomy is still the traditional method, and minimally invasive staging surgery is not widely available owing to limitations in exploring the full extent of the peritoneal surface, port-site metastasis, and a higher incidence of intraoperative tumor rupture [7,11,12]. The aims of this study were to introduce and evaluate the safety and feasibility of single-port laparoscopic surgical staging (SPLS), and to compare its perioperative surgical and survival outcomes with those of staging laparotomy in patients with early ovarian cancer.
Materials and methods
1. Data collection
We retrospectively reviewed the medical records of patients who underwent staging surgery for suspected ovarian cancer between January 1, 2014 and December 31, 2018 at Yonsei Cancer Center, Severance Hospital, Yonsei University College of Medicine in Seoul, Korea. During the study period, a total of 1,126 patients were diagnosed with ovarian cancer and underwent surgical staging, of whom 725 patients underwent staging laparotomy and 401 patients underwent staging laparoscopy. In the staging laparoscopy group, 149 patients underwent SPLS. There were 40 patients in the SPLS group and 41 patients in the laparotomy group who were diagnosed with International Federation of Gynecology and Obstetrics (FIGO) stage I ovarian cancer after surgical staging. Patients found to have metastatic cancers or borderline tumors in the final pathology were excluded. Only patients who underwent primary surgical staging at our hospital were included in this study (Fig. 1).

Flowchart of patient selection. SPLS, single-port laparoscopic surgical staging; FIGO, International Federation of Gynecology and Obstetrics.
The collected data included patient age, body mass index (BMI), pelvic adhesion, tumor size confirmed with preoperative imaging, tumor histology and grade, preoperative clinical stage, FIGO stage, operation time, estimated blood loss, hemoglobin change, transfusion rate, harvested lymph nodes (LNs), length of hospital stay, intraoperative and postoperative complications, postoperative pain scores (immediately postoperation, postoperative day 1, and postoperative day 3), postoperative adjuvant chemotherapy, and recurrence rate. Operative time was defined as the time from skin incision to completion of skin closure. Pain was evaluated using the numeric pain intensity scale (NPIS). Intraoperative complications were defined as adjacent organ or vessel injury, and wound complications were defined as wound discharge, dehiscence, and operative wound herniation. The number of cycles of postoperative adjuvant chemotherapy was compared. Follow-up duration was defined as the number of months from the operation date to the last follow-up date.
2. Surgical techniques
1) Single-port laparoscopic surgical staging
Under general anesthesia, the patient was placed in the lithotomy position and the skin was prepared in the usual manner. A uterine manipulator was used. As in our previous studies, after a 1.2–1.5 cm vertical intraumbilical skin incision with a 1.5–2.0 cm rectus fasciotomy was made, an Alexis wound retractor (Applied Medical, Rancho Santa Margarita, CA, USA) was inserted through the umbilical incision and a surgical glove was combined with the Alexis wound retractor for a single-port entry system (Fig. 2A). Subsequently, the abdominal cavity was filled with CO2 gas and the patient was placed in the Trendelenburg position [13,14].

Single-port laparoscopic surgical staging outside view (A) and safe tumor removal using a laparoscopic tissue retrieval bag (B, C).
After the pelvic and abdominal cavities were thoroughly explored, peritoneal washing cytology samples were obtained first. Thereafter, each procedure (e.g., hysterectomy, salpingo-oophorectomy, lymphadenectomy, omentectomy, peritonectomy, and appendectomy) was performed using bipolar or monopolar electrocautery and LigaSure (Covidien Valleylab, Boulder, CO, USA) or the Thunderbeat system (Olympus Medical Systems Corp., Tokyo, Japan) [4].
To avoid spilling into the intraperitoneal cavity, ovarian tumors were removed using a laparoscopic tissue retrieval bag (LapBag; Sejong Medical Co., Ltd., Seoul, Korea) (Fig. 2B and C). In the case of large tumors (longest diameter ≥15 cm), SW Kim’s technique was used to insert the tumors in a retrieval bag [15], the bag was retracted through the umbilicus, and the tumor was removed by aspirating and cutting it inside the bag. After hemostasis and irrigation, the Alexis wound retractor was removed and the umbilical fascia and subcutaneous layer were approximated with 1–0 and 2–0 Vicryl sutures (Ethicon, Piscataway, NJ, USA) [13,14].
2) Staging laparotomy
The preoperative preparation, surgical procedures, and postoperative management were essentially the same as for SPLS, except for a low midline or extended midline incision and nonuse of CO2 gas.
3. Statistical methods
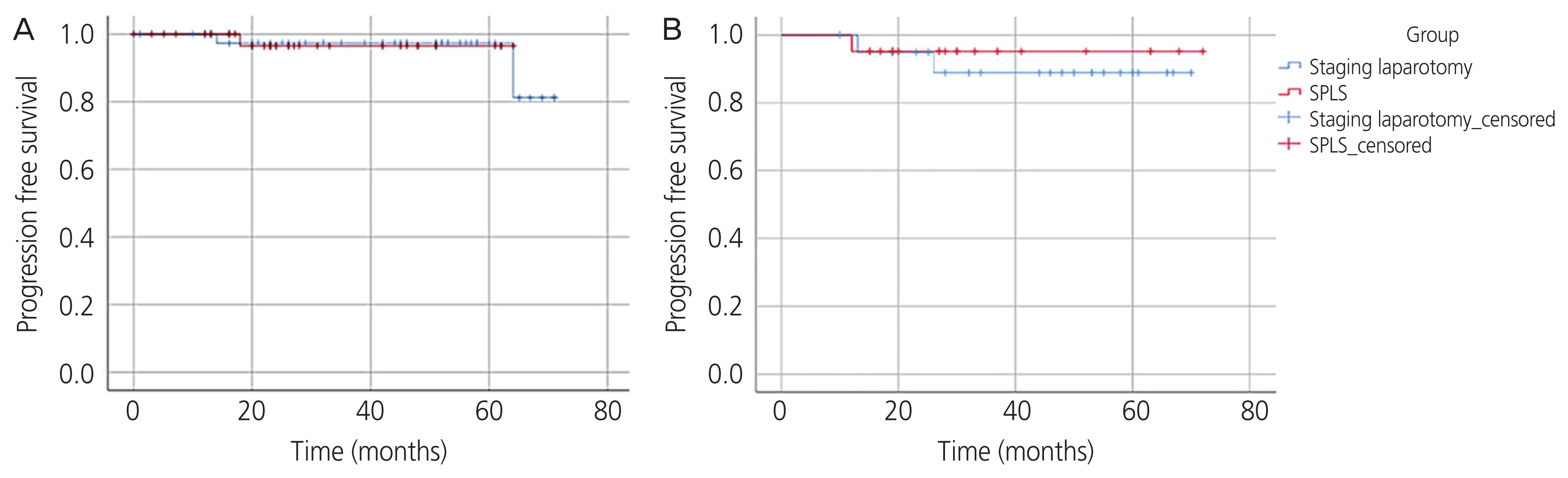
Statistical analysis was performed using SPSS version 25 (IBM, Armonk, NY, USA). A P-value of <0.05 was considered statistically significant. Categorical variables are presented as frequencies and percentages, and the Pearson χ2 test was used to evaluate differences between proportions. For continuous variables, medians and ranges of variables are presented, and the Mann-Whitney U test was performed. Kaplan-Meier estimates were used to evaluate differences in time to recurrence (Fig. 3A and B), and propensity score matching was used to adjust for selection bias (Supplementary Table 1) [16–18].
Results
In total, 81 patients who underwent surgical staging for early ovarian cancer were selected (40 SPLS and 41 laparotomy). In the SPLS group, 1 patient (2.5%) was converted to staging laparotomy because of a large tumor and 3 patients (7.5%) required an additional port.
The patient characteristics are shown in Table 1. The median age of patients in the SPLS group was younger than that of patients in the laparotomy group (46.4 [15–86] vs. 53.8 [19–61] years, P=0.005). There was no significant difference in median BMI (24.0 [15.0–36.8] vs. 23.2 [16.7–32.9] kg/m2, P=0.461) or pelvic adhesion rate (50.0% vs. 39.0%, P=0.320). The median tumor size was 11 cm in the SPLS group and 15.4 cm in the laparotomy group (P=0.001). The most common tumor histology type in the SPLS group was mucinous adenocarcinoma (30.0%), followed by clear cell carcinoma (25.0%), sex cord-stromal tumor (12.5%), and endometrioid adenocarcinoma (10.0%). In the laparotomy group, mucinous (22.0%) and endometrioid (22.0%) adenocarcinomas were the most common histologic types, followed by serous adenocarcinoma (19.5%) and clear cell carcinoma (19.5%). The tumor grade was not significantly different between the 2 groups (SPLS group vs. laparotomy group, grade 1: 27.5% vs. 17.1%, grade 2: 20.0% vs. 34.1%, grade 3: 25.0% vs. 24.4%; P=0.464). With respect to the FIGO stage, stage IA was the most common stage in both groups (70.0% and 39.0% in the SPLS and laparotomy groups, respectively), and the SPLS group had more stage IA cases (P=0.010). FIGO stage IC1 constituted 5.0% and 19.5%, IC2 20.0% and 22.0%, and IC3 2.5% and 19.5% in the SPLS and laparotomy groups, respectively.
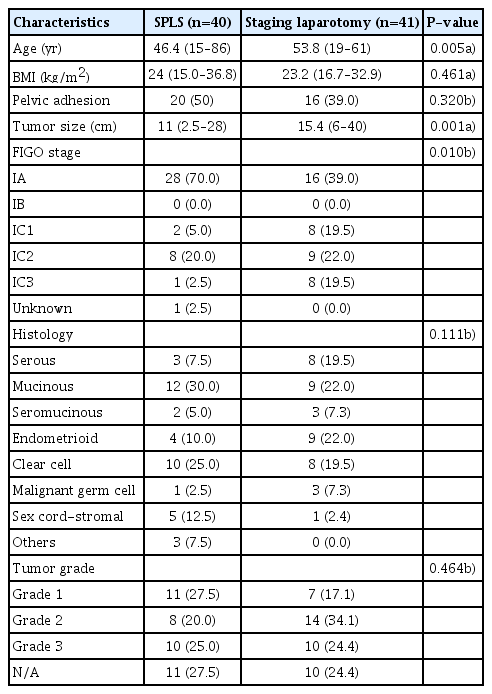
Patient characteristics in the single-port laparoscopic surgical staging (SPLS) and staging laparotomy groups (n=81)
Of 149 patients who underwent SPLS for suspected ovarian cancer, 96 patients were finally diagnosed with ovarian cancer after the surgery. Table 2 shows the preoperative clinical stage and postoperative FIGO stage of patients who were finally diagnosed with ovarian cancer. Fourteen patients (14.6%) were upstaged from preoperative clinical stage I to postoperative FIGO stage II to IV (FIGO stage II: 10 patients, stage III: 3 patients, and stage IV: 1 patient).
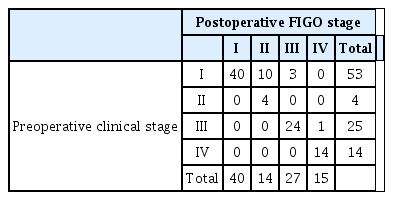
Comparison of preoperative clinical stage and postoperative surgical stage in patients who were diagnosed with ovarian cancer after single-port laparoscopic surgical staging (n=96)
Furthermore, we performed propensity score matching to minimize the difference in clinical characteristics between the two groups. The number of patients after propensity score matching decreased to 21 in each group, and the patient characteristics are summarized in Supplementary Table 1.
There was no significant difference between the 2 groups in terms of surgical procedures (Table 3). The number of harvested LNs (14.3 vs. 21.0, P=0.145), total operation time (201.4 vs. 203.0 minutes, P=0.806), and postoperative hemoglobin change (2.1 vs. 2.2 g/dL, P=0.981) did not differ between the SPLS and laparotomy groups. The estimated blood loss was smaller in the SPLS group (median: 102 mL, range: 10–1,100 mL) than in the laparotomy group (median: 371.5 mL, range: 10–1,870 mL) (P<0.001). Moreover, the intraoperative transfusion rate was lower in the SPLS group (1/40 [2.5%] vs. 8/41 [19.5%], P=0.015). The SPLS group showed lower postoperative pain score (immediately postoperation: 5.2 [2–7] vs. 6.0 [2–9], P=0.003; postoperative day 1: 3.7 [2–8] vs. 4.9 [2–10], P = 0.004; postoperative day 3: 2.9 [1–7] vs. 3.5 [2–7], P=0.017) and shorter postoperative hospital stay (5 vs. 9.5 days, P<0.001). The intraoperative major complication rate was similar between groups (2.5% vs. 4.9%, P=0.571). The SPLS group had 1 case of vascular injury, and the laparotomy group had 2 cases of bowel injury. There were no wound complications such as wound dehiscence or umbilical hernia in the SPLS group, whereas wound discharge was observed in 2 patients in the laparotomy group.
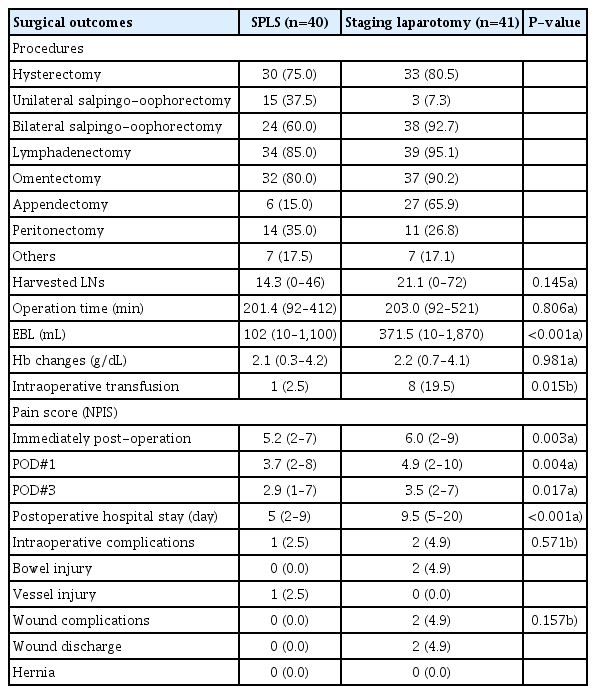
Surgical outcomes of single-port laparoscopic surgical staging (SPLS, n=40) and staging laparotomy (n=41)
The adjuvant chemotherapy rate was lower in the SPLS group than in the laparotomy group (40.0% vs. 75.6%, P=0.001) (Table 4). The median number of chemotherapy cycles was similar in the two groups (5.1 vs. 5.3 cycles, P=0.426). The SPLS group showed a longer time to chemotherapy after the surgery (17.3 vs. 15.3 days, P=0.031).
The median follow-up time was 35.8 months (range, 15–72 months) in the SPLS group and 48.2 months (range, 10–74 months) in the laparotomy group. There was no significant difference in recurrence rate (1/40 [2.5%] vs. 2/41 [4.9%], P=0.571). The values for progression-free survival were compared using Kaplan-Meier survival curves, and no significant difference was found between the two groups in all patients (P=0.945) (Fig. 3A) and after propensity score matching (P=0.594) (Fig. 3B). There were no deaths in either group during the study period.
Discussion
Many studies have compared open surgery and laparoscopic surgery in gynecology. Furthermore, several studies have compared conventional laparoscopy using multiple ports and laparotomy in ovarian cancer [9,18,19–21]. However, no previous study has compared single-port laparoscopic surgery and laparotomy in ovarian cancer, although several studies have compared single-port and multiport laparoscopic surgery for benign gynecologic diseases [13,22]. Therefore, this study is the first to investigate the feasibility of single-port laparoscopic surgery by comparing SPLS and staging laparotomy in early ovarian cancer.
According to our results, SPLS has several advantages in terms of surgical outcomes, such as lower estimated blood loss, lower transfusion rate, less postoperative pain, and shorter postoperative hospital stay, while showing no significant differences in survival outcomes. Less blood loss is well known as one of the main advantages of minimally invasive surgery. In this study, there was less blood loss in the SPLS group than in the laparotomy group; however, the change in hemoglobin after surgery did not differ between the 2 groups. This may be attributed to the increased transfusion rate during surgery in the staging laparotomy group. There were no significant differences in perioperative complications and postoperative wound complications.
The patients’ BMI and pelvic adhesion did not significantly affect the surgical methods used in this study. However, tumor size is usually a major concern in selecting laparoscopic surgery, especially in patients with possible malignant ovarian tumors. If the ovarian tumor is too large, it is usually difficult to remove safely, which is a limitation to performing laparoscopic surgery. Thus, most surgeons usually prefer laparotomy for large ovarian tumors. Nevertheless, there have been several attempts to perform minimally invasive surgeries even with large ovarian tumors, and we previously reported a technique for removing large ovarian tumors without aspiration or rupture in single-port laparoscopic surgery [15]. By using a large endoscopic bag, such as a 30×30 cm or 50×50 cm endoscopic tissue retrieval bag, huge ovarian tumors could be removed without any tumor spillage or cystic fluid aspiration. In this study, SPLS was successfully performed in most patients and showed a relatively lower tumor rupture rate than laparotomy (5% vs. 19.5%). However, in some patients, SPLS may require an additional port or conversion to laparotomy, especially when the tumor has severe adhesions.
The number of patients diagnosed with FIGO stage IA ovarian cancer was higher in the SPLS group. Accordingly, the postoperative adjuvant chemotherapy rate was lower in the SPLS group than in the laparotomy group. This may be associated with the relatively larger tumor size and higher tumor spillage rate in the laparotomy group. Although the laparotomy group had more patients with higher-stage disease in this study, there was no statistically significant difference in progression-free or overall survival rates between the 2 groups.
Our study was a retrospective study, and there may be some bias in the study design (i.e., review of electronic medical records). The sample size was too small to represent the entire population. A single surgeon performed all operations in the SPLS group, whereas multiple surgeons performed the operations in the staging laparotomy group. Each patient had a different duration of follow-up, and the follow-up period was <1 year in some patients; thus, there were insufficient data on cancer relapse or death. Further prospective randomized comparison studies are needed to confirm the safety of SPLS in terms of survival outcomes.
In conclusion, SPLS has several advantages in terms of blood loss, postoperative pain, and hospital stay, without compromising survival outcomes. SPLS, when performed by experienced gynecologic oncology surgeons, may be feasible and safe in the surgical staging of early ovarian cancer.
Notes
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Ethical approval
This study was approved by the Institutional Review Board of Severance Hospital (4-2020-0366).
Ethical approval
The Institutional Review Board approved the exemption of patients’ consent under the restriction that no identifiable personal information is revealed in the process.
Funding information
None.
Supplementary materials
Supplementary materials associated with this article can be found online at https://doi.org/10.5468/ogs.20216
Supplementary Table 1.
Patient characteristics of single-port laparoscopic surgical staging (SPLS) and staging laparotomy group after propensity score matching (n=42)