Gestational weight gain in twin pregnancies in Korea: application of the 2009 Institute of Medicine recommendations
Article information
Abstract
Objective
To investigate the effect of gestational weight gain (GWG) on maternal and neonatal outcomes based on the Institute of Medicine (IOM) guidelines for twin pregnancies.
Methods
This study included women with twin pregnancies who delivered at Seoul National University Bundang Hospital. Based on the weight gain per gestational week according to the 2009 IOM guidelines, the subjects were divided into the following 3 groups: inadequate, adequate, and excessive GWG. We compared the maternal and neonatal outcomes of each group.
Results
A total of 1,738 twin pregnancies were included in our study. Of these cases, 881, 694, and 163 (50.7%, 39.9%, and 9.4%, respectively) twin pregnancies were categorized into the inadequate, adequate, and excessive GWG groups, respectively. In the inadequate GWG group, the risks of preterm birth <34 weeks (aOR, 2.33, 95% confidence interval [CI], 1.63–3.34) and delivering neonates who were small for gestational age (aOR, 1.92, 95% CI, 1.42–2.60) were increased, and the risk of preeclampsia (aOR, 0.49, 95% CI, 0.32–0.76) was decreased. The excessive GWG group had an increased risk of the neonates being large for gestational age (aOR, 1.79, 95% CI, 1.15–2.81).
Conclusion
The 2009 IOM recommendations for GWG can be applied to Korean women with twin pregnancies to help achieve optimal maternal and neonatal outcomes. However, more than half of the women were categorized as having inadequate weight gain according to the guidelines. Further studies should be performed to obtain Korean national references for GWG in twin pregnancies.
Introduction
Appropriate gestational weight gain (GWG) is an important indicator of maternal and fetal well-being during pregnancy [1]. Excessive maternal weight gain leads to gestational hypertensive disorders and diabetes, increased risk of cesarean section, large for gestational age (LGA) infants, and higher postpartum weight retention, while inadequate weight gain could be the cause of preterm birth and infants who are small for gestational age (SGA) [2-5]. To highlight and suggest adequate weight gain goals during pregnancy, recommendations for GWG were published by the Institute of Medicine (IOM), now renamed the National Academy of Medicine, in 1990 [6]. According to the guidelines, a total GWG of 11.5–16 kg is considered adequate for women of normal weight with singleton pregnancies. Many studies have revealed that weight gain outside of these guidelines in singleton pregnancies is associated with adverse pregnancy outcomes [7].
The IOM committee also provided a recommendation for adequate GWG in twin pregnancies [6]. However, it is difficult to directly apply these guidelines to Korean women for the following reasons. First, the guidelines published by the IOM are solely based on the data of American women [8]. Since GWG may be influenced by various factors, which may be social, environmental, or genetic, the values for appropriate GWG are different for each ethnic group [9-13]. Second, the World Health Organization (WHO) has set the cutoff for the overweight body mass index (BMI) category at 23 for the Asian population, which is different from Western standards [14]. Third, there is a lack of research addressing whether it is appropriate to apply these guidelines to Korean women with twin pregnancies.
To address these issues, the purpose of this study is to investigate the effect of GWG on maternal and neonatal outcomes based on the IOM guidelines for Korean women with twin pregnancies and to determine whether these guidelines can be applied to this group of women.
Materials and methods
1. Study population and study design
This retrospective study included women with twin pregnancies who delivered at Seoul National University Bundang Hospital between January 2005 and June 2019. Among them, cases with circumstances that may have affected the GWG, such as single fetal demise, delivery before 24 weeks of gestation, delayed interval delivery, major fetal anomalies, and cases with an unknown pre-pregnancy BMI and GWG; and cases with twin-specific complications, such as severely weight discordant twins (>30%), twin-to-twin transfusion syndrome (TTTS), and monochorionic monoamniotic (MCMA) twins, were excluded from the analysis. Based on the weight gain per gestational week according to the 2009 IOM guidelines, the cases were divided into the following 3 groups: inadequate, adequate, and excessive GWG. We compared the maternal and neonatal outcomes of each group and calculated the adjusted odds ratios (aORs) for each outcome, which were then compared to the reference group (adequate GWG group) by multiple logistic regression analysis after adjusting for confounding factors.
2. Setting of the inadequate, adequate, and excessive gestational weight gain groups
The rate of weight gain (kg/week) in each mother was calculated by dividing the total gestational weight by the gestational age at delivery in weeks. According to the method proposed by Fox et al. [15,16], an adequate GWG per gestational week was defined as the value obtained by dividing the IOM recommended weight gain by 37 weeks. The value was dependent on the pre-pregnancy BMI group, as specified in Supplementary Table 1. For example, if a woman who delivered at 38 weeks had a 30 kg weight increase in pregnancy, her rate of weight gain would be 30 kg/38 weeks (i.e., 0.789 kg/week). If the mother’s pre-pregnancy BMI was within the normal range, adequate GWG would be 0.454–0.662 kg/week. If the calculated value was greater than 0.662 kg/week, the mother would be classified into the excessive GWG group.
3. Clinical factors, maternal outcomes, and neonatal outcomes
The baseline characteristics in each pregnancy including the maternal age, height, pre-pregnancy weight, parity, chorionicity, and presence of preexisting such as hypertension (HTN) and diabetes mellitus (DM) were evaluated at the initial prenatal visit. Because it is common to visit the outpatient clinic after the first trimester, we asked the mothers for their prepregnancy weight and recorded it. The pre-pregnancy BMI was calculated as the maternal pre-pregnancy weight (kg)/height (m2). We used the BMI classification for an Asian population according to the WHO [14]. The categories of underweight, normal weight, overweight, and obese were defined as BMI <18.5 kg/m2, 18.5–23 kg/m2, 23–25 kg/m2, and ≥25 kg/m2, respectively.
When the mother was hospitalized for delivery, the predelivery weight was measured. The total gestational weight was calculated by subtracting the self-reported pre-pregnancy weight from the pre-delivery weight. Each case’s medical charts were reviewed to obtain the delivery method and complications during pregnancy, including preterm labor, preterm premature rupture of membranes (PPROM), HTN, preeclampsia (PE), and gestational DM (GDM). HTN during pregnancy included gestational HTN, PE, eclampsia, and superimposed PE. The mode of delivery for women with twin pregnancies was decided after consulting with the mothers. Essentially, if both fetuses had vertex presentations, vaginal deliveries could be attempted. The neonatal outcomes included the birth weight, whether the infant was SGA or LGA, neonatal intensive care unit (NICU) admission, and low Apgar scores (Apgar score <7 at 1 minute or 5 minutes). We defined SGA as a weight lower than the 10th percentile for the gestational age and LGA as a weight greater than the 90th percentile for the gestational age, based on the Korean twin growth curves [17].
4. Statistical analysis
Continuous data are presented as mean±standard deviation, and categorical data are presented as number (%). To compare the 3 groups, analysis of variance was used for continuous variables and the χ2 test or Fisher’s exact test was used for categorical variables. Using the adequate GWG group as the reference group for comparison, the unadjusted OR and aOR for each maternal outcome in the inadequate and excessive GWG groups were calculated using univariate and multivariate logistic regression analysis, respectively. For the analysis of the neonatal outcomes, a generalized estimating equation (GEE) was used to adjust for the familial correlation between twins from a single mother. The Firth correction was used to calculate the aOR for rare-event data [18]. A P-value of <0.05 was considered statistically significant. IBM SPSS Statistics version 23.0 software (IBM Inc., Armonk, NY, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA) were used for the analyses.
Results
A total of 2,088 women with twin pregnancies gave birth at Seoul National University Bundang Hospital between 2005 and 2019. Among these women, 350 women (45 with a single fetal demise, 33 who delivered before 24 weeks of gestation, 5 with delayed interval delivery, 8 with major fetal anomalies, 106 without pre-pregnancy BMI data, 9 who could not calculate their GWG, 123 with severely discordant twins, 21 with TTTS, and 2 with MCMA twins) were excluded from the final analysis. Of the 1,738 patients who met the inclusion criteria, 881, 694, and 163 (50.7%, 39.9%, and 9.4%, respectively) were categorized into the inadequate, adequate, and excessive GWG groups, respectively. The distribution of the GWG for each BMI category is detailed in Fig. 1.
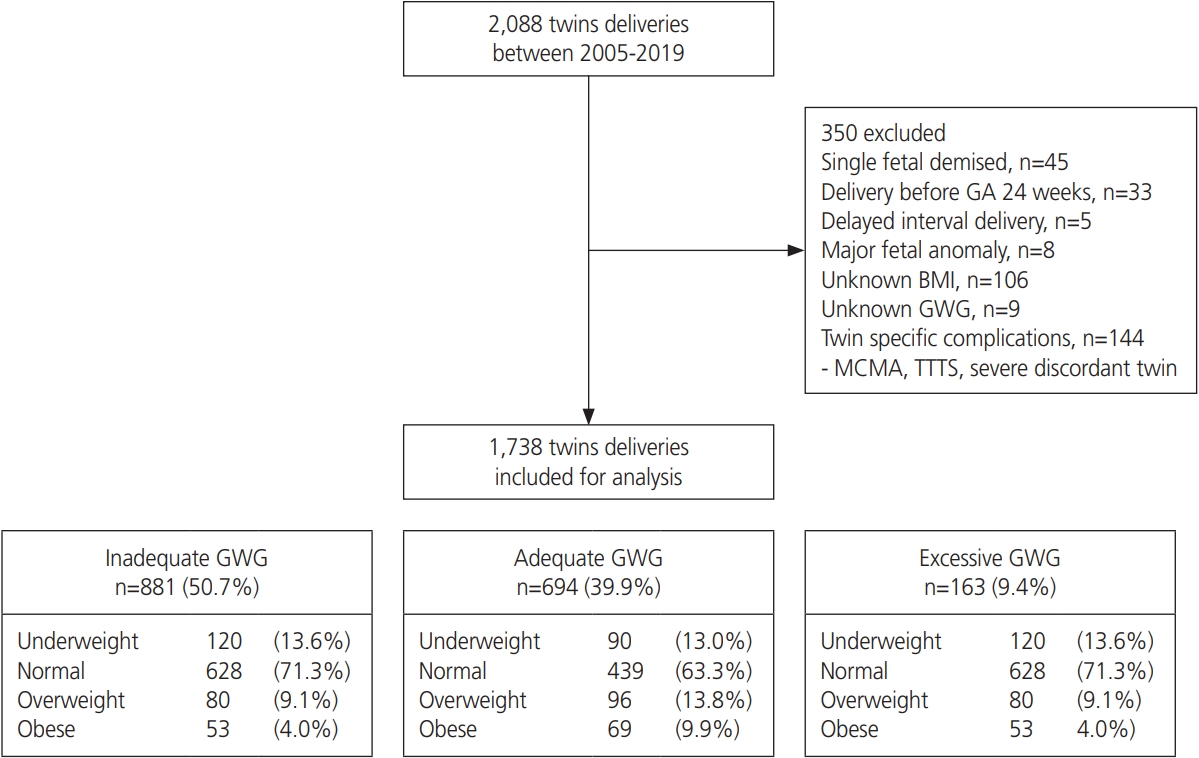
Study flow chart and population. GA, gestational age; BMI, body mass index; GWG, gestational weight gain; MCMA, monochorionic monoamniotic twin; TTTS, twin-to-twin transfusion syndrome.
Table 1 presents the demographic characteristics, and maternal and neonatal outcomes for each group. The maternal age, height, pre-pregnancy weight, and BMI were different between the 3 groups. The maternal age was highest in the inadequate GWG group; and the height, pre-pregnancy weight, and BMI were highest in the excessive GWG group (P<0.001). The excessive GWG group had a higher prevalence of preexisting DM than the other 2 groups (P=0.029). There were no significant differences in the prevalence of nulliparity, dichorionicity, and preexisting HTN between the 3 groups.
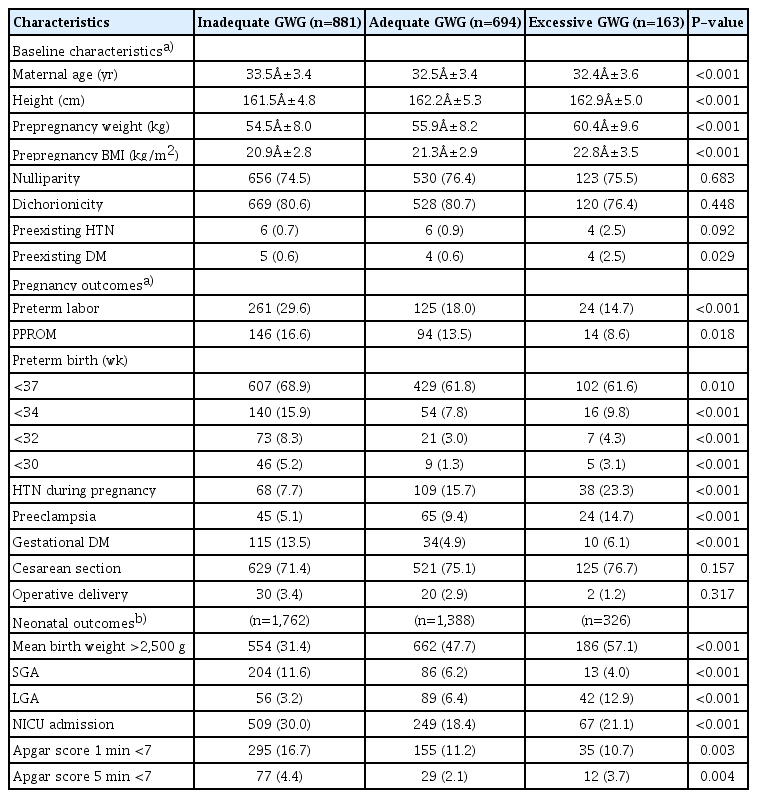
The baseline characteristics, pregnancy and neonatal outcomes according to the gestational weight gain (GWG) groups
There were significantly higher incidences of preterm labor, PPROM, preterm birth, and GDM in the inadequate GWG group than in the other 2 groups (all P<0.001 except for PPROM [P=0.018]). However, the prevalences of HTN during pregnancy and PE were highest in the excessive GWG group (all P<0.001). The mode of birth differed slightly between the 3 groups; for example, cesarean section was more prevalent in the excessive GWG group and operative deliveries were more common in the inadequate GWG group; however, the results were statistically insignificant.
Regarding neonatal outcomes, the incidences of infants who were SGA, required NICU admission, or had Apgar scores <7 at 1 minute and 5 minutes were highest in the inadequate GWG group; the proportions of infants who had a mean birth weight >2,500 g and were LGA were highest in the excessive GWG group (all P<0.001 except Apgar scores <7 at 1 minute [P=0.003] and 5 minutes [P=0.004]). The adequate GWG group showed the lowest NICU admission rate (18.4% vs. 30.0% in the inadequate GWG group and 21.1% in the excessive GWG group).
We performed multivariate logistic regression analysis using variables that showed significant differences between the 3 groups. The results of the maternal and neonatal outcomes are summarized in Tables 2 and 3, respectively. The patients in the inadequate GWG group were more likely to have preterm labor (aOR, 1.96, 95% confidence interval [CI], 1.52–2.53), preterm birth (<37 weeks, aOR, 1.39, 95% CI, 1.11–1.73; <34 weeks, aOR, 2.33, 95% CI, 1.63–3.34; <32 weeks, aOR, 2.95, 95% CI, 1.74–5.01; and <30 weeks, aOR, 4.47, 95% CI, 2.06–9.67), and GDM (aOR, 3.38, 95% CI, 2.17–5.27), even after adjusting for all confounders. In contrast, the likelihoods of developing HTN during pregnancy and PE (aOR, 0.43 and 0.49, 95% CI, 0.30–0.60 and 0.32–0.76, respectively) were lower in the inadequate GWG group. There were no significant associations between each maternal outcome and excessive GWG (Table 2).

The risks for the maternal outcomes in the inadequate and excessive gestational weight gain (GWG) groups compared to the adequate GWG group (reference group)
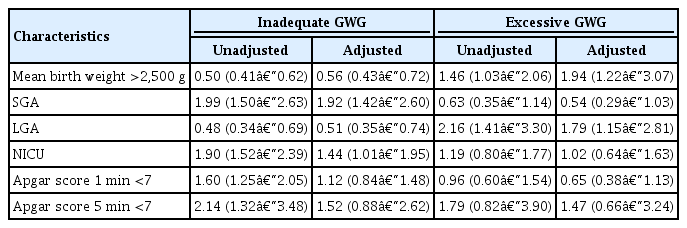
The risks for the neonatal outcomes in the inadequate and excessive gestational weight gain (GWG) groups compared to the adequate GWG group (reference group)
The neonates of mothers in the inadequate GWG group were more likely to be SGA and require admission to the NICU (aOR, 1.92 and 1.44, 95% CI, 1.42–2.60 and 1.01–1.95, respectively). Twins of mothers with excessive GWG tended to be LGA (aOR, 1.79, 95% CI, 1.15–2.81). The odds of lower Apgar scores were higher in the inadequate GWG group; however, they were not significant after considering other confounders (Table 3).
Table 4 presents the aORs for the major maternal and neonatal outcomes according to the BMI and GWG categories. The risks of major outcomes in women who had inadequate GWG and BMIs under 23 kg/m2 were similar to those in the total population. In women with BMIs over 23 kg/m2, only preterm labor and HTN during pregnancy were associated with inadequate GWG. In particular, there was a higher risk of preterm birth before 30 weeks in women with both excessive GWG and BMIs under 23 kg/m2 (aOR, 9.11, 95% CI, 1.49–55.76), which was different from the original results showing no association between preterm birth and excessive GWG. Women with excessive GWG and BMIs over 23 kg/m2 showed an increased risk of the infants being LGA and a decreased risk of the infants being of SGA.
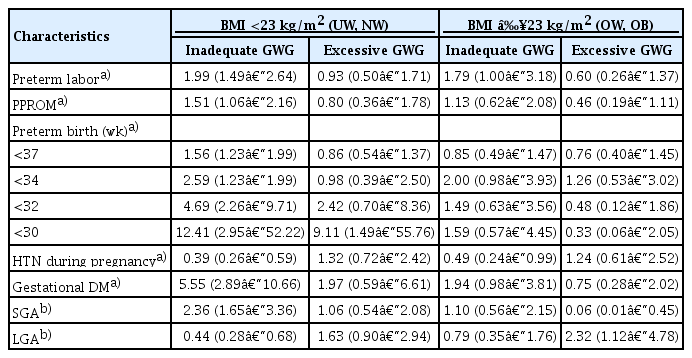
The risks for the maternal and neonatal outcomes in the inadequate and excessive gestational weight gain (GWG) groups compared to the adequate GWG group (reference group): subgroup analysis according to the body mass index (BMI) categorization (cut-off: 23 kg/m2)
Sub-group analysis was conducted to evaluate the etiologies of preterm birth according to the GWG groups of the women who gave birth before 34 weeks (Table 5). According to the GWG groups, the frequency of spontaneous preterm birth (due to preterm labor or PPROM) showed a linear association, which was the highest in the inadequate GWG group (80% vs. 75.9% vs. 46.7% for inadequate vs. adequate vs. excessive GWG, P<0.05).
Discussion
According to the results of this study, in terms of the maternal outcomes, women with inadequate GWG had a higher risk of preterm birth and preterm labor, and a lower risk of HTN during pregnancy, including PE, than women with adequate GWG.
Preterm birth is the most important obstetric outcome. Many previous studies have investigated this subject and have found an association between GWG and preterm birth [15,19-22]. However, the results are conflicting. A strong association between GWG and preterm birth was first demonstrated by Fox et al. [15]. According to their study, women with inadequate GWG had an incidence of preterm birth that was 3 times higher than women with normal weight gain. Subsequent studies have also shown an increased risk of preterm birth in women with low GWG [15,19,23]. However, some studies have reported that there is no association between GWG and preterm birth [20,21]. These conflicting results might have stemmed from differences in the research populations and methods. In our study, most of the study population comprised Korean women who had delivered in the past 14 years. Such a large monoethnic population can provide more accurate results about the relationship between GWG and preterm birth in modern Korean mothers with twin pregnancies. According to our results, after subgroup analysis according to the BMI categorization, the risk of preterm birth was increased in women with inadequate GW, in those who had normal weights (BMI under 23 kg/m2), and in those with excessive GWG. These results were consistent with those in large studies conducted for singleton pregnancies in Korea and China [12,24]. Taken together, both inadequate and excessive GWG might be risk factors for preterm birth.
According to the subgroup analysis of women with preterm births, the rate of spontaneous preterm birth (preterm labor and PPROM) was increased, but that of medically indicated preterm birth (HTN, SGA, etc.) was decreased in the inadequate GWG group. In addition, the etiologies of preterm birth showed a linear association according to the GWG group. Fox et al. [15] and Kosinska-Kaczynska et al. [21] reported similar results to those in our study. Based on these results, the increase in preterm birth in women with inadequate GWG was attributed more to preterm labor than to SGA.
Many studies have reported that excess GWG can increase the risk of GDM [25-27]. However, our study showed that the proportion of women with GDM was higher in the inadequate GWG group. Women who were diagnosed with GDM tended to control their weight more than others; thus, women with GDM were likely to have been assigned to the inadequate GWG group. Similar findings were reported by Lee et al. [28]. As lower GWG is a crucial factor for optimal outcomes in mothers with GDM [29,30], these women strive to reduce their GWG. According to a study by Katon et al. [31], women with GDM gained weight at an average of 9.1 kg before being diagnosed with GDM, but after diagnosis the average weight gain was 2.02 kg. These findings support our results. However, we have considered that further analysis and investigations are required to evaluate these contradictory results.
Regarding the neonatal outcomes, when compared to the normal reference population, neonates who were born to women with inadequate GWG had a higher risk of being SGA, and those born to women with excessive GWG had a higher risk of being LGA. Previous studies have already reported an association between inadequate GWG and neonates who are SGA, and excessive GWG and neonates who are LGA [32-35]. Li et al. [32] analyzed the data from 33,973 pregnancies in the Chinese population and reported that the aORs for LGA infants with excessive GWG and SGA infants with inadequate GWG were 2.32 and 1.41, respectively (95% CI, 2.12–2.53 and 1.26–1.57, respectively). Another study of 1,482 twins indicated that the inadequate GWG group had a higher risk of being SGA (aOR, 1.44; 95% CI, 1.01–2.06) [35]. Our results were consistent with previous study findings. These findings are meaningful for Korean women with twin pregnancies as they indicate that the IOM guidelines may be used appropriately for counseling about the weight of newborn babies.
There are several risk factors for adverse maternal and infant outcomes. Some of these risk factors, such as GWG, are modifiable, meaning they can be changed to help reduce risk. Several studies reported that having a weight gain goal played an important part in gaining an appropriate amount of gestational weight. Therefore, physicians should suggest lifestyle interventions to patients for optimal GWG.
To our knowledge, this study included the largest study population in a recent period to evaluate whether the IOM guidelines can be applied to Korean women with twin pregnancies. In addition, our study provided reliable results through multivariate analysis of factors such as maternal outcomes through binary logistic regression and neonatal outcomes by the GEE. Although the gestational age at birth, chorionicity, and fetal sex are important variables that can lead to adverse outcomes, many previous studies did not correct for them [34,36-39]. In addition, GEE analysis, which is a method used to adjust for the correlation between twins, was not performed in previous studies despite its necessity [22,34,36,39].
However, there are some limitations to this study. Due to the retrospective nature of our study, we calculated GWG/week using the methods proposed by Fox et al. [15]. It is a simple method, but it cannot provide the exact GWG/week because the increase in the rate of weight gain in the 1st trimester is different from that in 2nd and 3rd trimesters [40]. Therefore, it is necessary to identify the relationship between weight gain and outcomes by separating the weight gain in the 1st trimester from that after the 2nd trimester. When we addressed weight gain issues, we did not consider caloric excess and fluid overload. To do this, the maternal diet and amount of generalized edema need to be assessed, but data on these factors could not be obtained due to the retrospective nature of the study. In addition, there were not enough women in the excessive GWG group to obtain significant findings. Moreover, a large number of the women were classified into the inadequate GWG group. Furthermore, although an association between GWG and adverse outcomes was identified, a cause-and-effect association was not clear. This means that it was unclear if the baby was small because of low GWG or if the GWG was low because the baby was small. Further prospective studies are necessary to analyze this association and set a clear standard for GWG while considering this point, as well as to set an appropriate standard for weight gain specifically for Korean women with twin pregnancies.
Notes
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Ethical approval
The Institutional Review Board of Seoul National Bundang University Hospital approved the collection of the information for this study (B-1907-553-101). We followed the ethical standards for human experimentation established in the Declaration of Helsinki.
Supplementary materials
Supplementary materials associated with this article can be found online at https://doi.org/10.5468/ogs.20133
Supplementary Table 1.
The reference value of gestational weight gain (GWG) by the Institute of Medicine (IOM)

