|
 |
- Search
| Obstet Gynecol Sci > Volume 57(2); 2014 > Article |
Abstract
Objective
The purpose of our study was to investigate the effect of the mode of delivery on the oxidant and antioxidant system in umbilical cord blood.
Methods
We performed gas analysis of umbilical venous blood and umbilical arterial blood immediately after delivery in 38 women; eighteen women had a vaginal delivery while 20 women delivered via cesarean section at over 37 weeks gestation. We examined lipid peroxide concentration by thiobarbituric acid reaction, protein carbonyl content by 2,4-dinitrophenylhydrazine reaction, and total antioxidant capacity by oxygen radical absorbance capacity assay.
Results
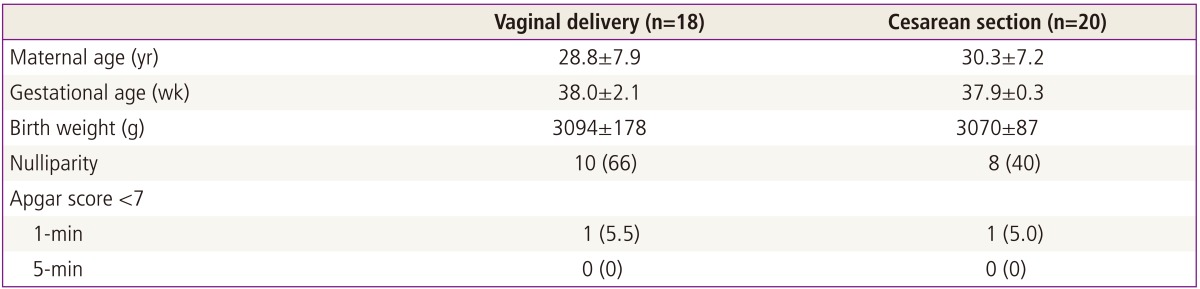
Lipid peroxide levels in umbilical venous blood were significantly higher in patients delivering by planned cesarean section (1.81 ┬▒ 0.06 nmol/mg protein) than those with vaginal delivery (1.24 ┬▒ 0.05 nmol/mg protein) (P < 0.05). Antioxidant capacity in umbilical venous blood was significantly higher in patients delivering by planned cesarean section (119.70 ┬▒ 0.13 ┬ĄM/┬ĄL) than those with a vaginal delivery (118.70 ┬▒ 0.29 ┬ĄM/┬ĄL) (P < 0.05). There was no significant difference in the carbonyl content of umbilical venous blood or in the lipid peroxide, carbonyl content, and total antioxidant capacity of umbilical arterial blood.
Conclusion
Lipid peroxidation levels and antioxidant capacity in umbilical venous blood were higher in patients delivering by planned cesarean section than those with a vaginal delivery. Therefore, we propose that both the mother and neonate are exposed to higher oxidative stress during cesarean section delivery.
Oxidative stress is defined as an imbalance between pro-oxidant and antioxidant forces resulting in an overall pro-oxidant insult. Oxygen-derived reactants, collectively termed reactive oxygen species (ROS), are a normal byproduct of cellular metabolism; as such, cells also contain a natural antioxidant defense system [1,2]. The body contain endogenous antioxidants such as superoxide dismutase, glutathione peroxidase, and bilirubin while total antioxidant capacity is also determined by the exogenous molecules such as vitamin C, vitamin E.
Metabolic activation of molecular oxygen frequently gives rise to ROS; however, when the protective capacity level of antioxidants has been exceeded, these products can become toxic to most cellular components, as they stimulate lipid peroxidation and protein inactivation [3]. The resulting damage is referred to as oxidative stress. Oxidative stress can be assessed in vivo using the plasma biomarkers malondialdehyde and protein carbonyl.
In women with normal, uncomplicated pregnancies lipid peroxide and antioxidant levels are elevated as compared to non-pregnant women; this increase is proportional to gestational length [4]. The placenta is a major source of oxidative stress during pregnancy. The placenta is rich in polyunsaturated fatty acids, and it is an abundant source of lipid peroxides which are secreted into the maternal circulation. In normal pregnancy, placental lipid production is believed to be kept under control by placental antioxidant enzymes [5]. When compared to women with normal pregnancies, women with complicating factors such as diabetes mellitus, preeclampsia, and preterm labor experience ever-increasing levels of oxidative stress and reduced antioxidant capacity [6].
The increase in oxidative stress during complicated pregnancy has an effect on neonatal outcome, leading to various short- and long-term problems in neonates such as retinopathy, bronchopulmonary dysplasia, intraventricular hemorrhage, and necrotizing enterocolitis, especially in premature babies [7,8,9,10]. Pulmonary oxidative stress often occurs in humans during acute lung injury and in acute respiratory distress syndrome [11]. These increment of oxidative stress markers also reported in perinatal asphyxia and hypoxic ischemic encephalopathy in term infants [12].
During parturition, oxidative stress increases more profoundly. Increasing energy demand and metabolic activity by the contraction of skeletal muscle during any type of exercise, combined with a rise in using oxygen, is known to result in increased levels of ROS. As labor involves a series of contractions involving both skeletal muscle and uterine smooth muscle, we expect that oxidative stress will increase during vaginal delivery (VD) as compared to a planned cesarean section (CS).
Despite the well-known adverse effects of oxidative stress on the mother, fetus, and newborn, the effects of the type of delivery on the oxidative stress experienced by both mother and child are still not clear. The purpose of our study is to investigate the effects of the mode of parturition on the oxidant and antioxidant system via umbilical cord blood analysis and on neonatal outcomes.
Overall, thirty-eight women with pregnancies between 37 and 41 weeks of gestation and their newborns were investigated. The participants were divided into two groups according to the mode of their labor and delivery: group VD (n=18) and group CS (n=20). Gestational age was determined based on the menstrual history or obstetrical findings.
Planned cesarean delivery is used as a proxy for cesarean delivery on maternal request since these women are planning to undergo a cesarean, although the reason may be breech presentation or previous uterine surgery include CS for some women and maternal desire for others. Mothers who delivered via emergency CS, surgery after prolonged labor, or had gestational problems such as oligohydramnios, eclampsia/preeclampsia, diabetes mellitus, or preterm labor might have increased levels of oxidative stress due to reasons beyond the mode of delivery and thus were excluded. Further details are summarized in Table 1.
The material used in the study was arterial and venous cord blood. Umbilical cord blood samples were obtained immediately after delivery, before delivery of the placenta, and collected in a tube without anticoagulant. Tubes were centrifuged at 2,000 ├Śg for 10 minutes at 4Ōäā to collect the serum; samples were stored at -80Ōäā until analysis.
Lipid perioxidation was measured using the thiobarbituric acid reactionas described by Ohkawa et al. [13]. When thiobarbituric acid is applied to a mixture of acetaldehyde and sucrose, a 532 nm-absorbing chromogen is produced which is indistinguishable from that formed by the malondialdehyde and thiobarbituric acid adduct. The malondialdehyde assay is the most generally used test in the appreciation of the role of oxidative stress in disease. The results are expressed as nmol of malondialdehyde incorporated/mg of protein based on an average absorptivity (electromagnetic wave=1.56├Ś105).
Measurement of protein oxidation was determined by assay for protein carbonyl content using the 2,4-dinitrophenyl hydrazine reaction. Serum samples were divided into two equal aliquots containing approximately 1.0 mg of protein each. Both aliquots were precipitated with 10% trichloroacetic acid (w/v, final concentration). One sample was treated with 2N HCl, and the other sample was treated with an equal volume of 0.2% (w/v) dinitrophenylhydrazine (DNPH) in 2 N HCI. Both samples were incubated at 25Ōäā in 15-mL conical glass centrifuge tubes and stirred at 5-minutes intervals. The samples were then re-precipitated with 10% trichloroacetic acid (final concentration) and subsequently extracted with ethanol/ethyl acetate (l:l, v/v) and then re-precipitated at 10% trichloroacetic acid. The pellets were carefully drained and dissolved in 6M guanidine HCI in 20 mM sodium phosphate buffer, pH 6.5. Insoluble debris was removed by centrifugation at 6000 ├Śg at 4Ōäā. The difference between the spectra of the DNPH-treated sample versus the HCl control was determined and the results are expressed as nmol of DNPH incorporated/mg of protein based on an average absorptivity of 21.0 mM-1 cm-1 for most aliphatic hydrazones [14].
Total antioxidant capacity was measured by oxygen radical absorbance capacity (ORAC) assay kit (Zen-Bio, Research Triangle Park, NC, USA).The ORAC assay was carried out at 37Ōäā on a spectrofluorometer at an excitation wavelength of 485 nm and an emission wavelength of 528 to 538 nm (cutoff, 530 nm). The procedure was based on the method reported by Cao et al. [15].
All data were presented as mean┬▒standard deviation. The comparison of parameters were performed using an independent t-test. P<0.05 was considered statistically significant. Data were analyzed using the IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). At a 2-sided, significance level at 0.05, we calculated a sample size of 15 patients would be necessary to demonstrate the difference between two groups cohort at 95% power. The sample size was increased to 20 patients in planned CS group (G-Power 3.1.5).
As shown in Table 1, the age of the mother, fetal gestational age at delivery, birth weight, and apgar score did not differ among groups (P > 0.05). Lipid peroxide levels in umbilical venous blood were significantly higher in patients delivering by planned CS (1.81┬▒0.06 nmol/mg protein) as compared to those who delivered vaginally (1.24 ┬▒ 0.05 nmol/mg protein) (P < 0.05) (Fig. 1). Antioxidant capacity in umbilical venous blood was significantly higher in patients delivering by planned CS (119.70 ┬▒ 0.13 ┬ĄM/┬ĄL) than those with a VD (118.70 ┬▒ 0.29 ┬ĄM/┬ĄL) (P < 0.05) (Fig. 2). There was no significant difference in the carbonyl content of umbilical venous blood (CS, 5.26 ┬▒ 0.76 nmol/mg protein; VD, 5.14 ┬▒ 0.10 nmol/mg protein) or in lipid peroxide (CS, 1.87 ┬▒ 0.15 nmol/mg protein; VD, 1.80 ┬▒ 0.13 nmol/mg protein) , carbonyl content (CS, 5.56 ┬▒ 0.14 nmol/mg protein; VD, 5.43 ┬▒ 0.10 nmol/mg protein), and total antioxidant capacity (CS, 119.12 ┬▒ 0.27 ┬ĄM/┬ĄL; VD, 119.04 ┬▒ 0.33 ┬ĄM/┬ĄL) of umbilical arterial blood (Figs. 2, 3).
Aerobic respiration generates adenosine triphosphate in the mitochondria (oxidative metabolism) and as a result, several compounds are produced. Most of these compounds are beneficial; however, a small percentage (<5%) can cause cellular injury if their concentration becomes sufficiently elevated [16].
Source of internal oxidative stress include peroxisomes and enzymes including xanthine oxidase and the nicotinamide adenine dinucleotide oxidase complex. External sources of oxidative stress include radiation, aerobic exercise, chemical compounds such as environmental pollutants, smoking and alcohol. Reactive species can be classified into four groups based on the main atom involved: ROS, reactive nitrogen species, reactive sulfur species, and reactive chloride species [17].
Of all the compounds derived from oxidative metabolism, ROS are the most abundantly produced. ROS include the superoxide anion, hydrogen peroxide, and hydroxyl radical [18]. When oxygen-derived reactants are overproduced, they are important mediators of cell and tissue injury as they can cause oxidation of lipids, proteins, and polysaccharides and as well as DNA damage; therefore, ROS can have a wide range of biologically toxic effects.
Recently, ROS have been reported to play an important role in the progression of certain cardiovascular diseases, stroke, Alzheimer disease, and certain types of cancer [19,20,21,22,23]. Pregnancy is a physiological state accompanied by high energy demands of many bodily functions and an increased oxygen requirement. This augmented oxygen requirement increases the rate of production of ROS which can damage the fetus. It has been reported that oxidative stress and a disrupted antioxidant system are involved in a variety of pregnancy complications such as preterm labor, fetal growth restriction, preeclampsia, and miscarriage [24,25].
Infants have less protection against oxidation. In comparison with healthy adults, lower levels of plasma antioxidants such as vitamin E, ╬▓-carotene, melatonin, and sulfhydryl groups as well as lower levels of plasma metal binding proteins such as ceruloplasmin and transferrin, and reduced activity of erythrocyte superoxide dismutase are typical of newborn infants [26,27,28].
Oxidative stress cause damage to the cell membrane, leading to various short- and long-term problems in neonates such as retinopathy, bronchopulmonary dysplasia, intraventricular hemorrhage [7,8,9,10]. Recently, free oxygen radicals have been reported to play an important role in necrotizing enterocolitis in premature infants [29] and oxidative stress markers have been correlated with higher levels of mortality in infants with hypoxic ischemic encephalopathy [12].
Various maternal, fetal, and environmental factors increase oxidative stress during birth. Both skeletal and uterine muscles participate during labor and delivery. It has been documented that exercise can increase the generation of ROS and this is especially true for single bouts of exercise. As a consequence of increased concentration of ROS, oxidative damage of lipids, proteins and DNA have been reported following single bouts of exercise [30]. It has been demonstrated that regular exercise increases the activity of proteasome complex in the myocardium, and decreases the level of carbonylated proteins; however, prolonged or short-term sub-maximal aerobic exercise has been demonstrated to induce oxidative stress since recovery, which is obligatory for efficient stress responses, is missing [31]. All these data lead to the hypothesis that oxidative stress may be present during labor and VD.
But the hypothesis is controversial. Yaacobi et al. [3] reported that malondialdhyde concentration is higher in VD and emergency CS after prolonged labor group as compared elective CS without labor. However, Mutlu et al. [32] reported conflicting result. Their study demonstrated CS increases total oxidative stress, an oxidative stress index, and lipid hydroperoxide level.
Our results clearly show that oxidative stress and antioxidant defense system were profoundly modulated by the way of delivery. There is ample evidence to suggest that regular exercise increases the activity of antioxidant enzymes and increased levels of ROS appear to be necessary during the exercise session. In addition to antioxidant enzymes, the oxidative damage repair systems are important to minimize the dangerous effects of ROS [1,2,3,4,6]. High oxidative stress markers indicates that the balance is disturbed in favor of oxidants and increased antioxidants are not enough to compensate the oxidative stress generated in paturients. Our results showed higher levels of oxidative stress markers in the cesarean delivery group even though antioxidant capacity was also increased.
Several factors may have contributed to the formation of free radicals in CS group. Inspired oxygen during anaesthesia may be a factor. Khaw et al. [33] repoted that high inspired oxygen fraction caused increase in oxygen free radical activity in maternal blood. And surgery itself may possibly have contributed to the lipid peroxidation, although an effect as large as this was not seen in some study [34]. Further studies to investigate the origin of the free radical may be performed.
This study has a number of limitations. First, the number of participants (n = 38) is small. Secondly, we were unable follow-up with the infants to obtain long-term outcome. There is no significant difference of Apgar score and acidity in blood gas analysis of both groups. Perhaps the rate of short-term outcomes did not differ significantly between the groups. But the relationship between oxidant/antioxidant status and the mode of delivery and/or influence on neonatal morbidity remains to be determined by studies with a larger sample size and long-term follow up in order to clarify this issue. Third, we conducted uncomplicated pregnancy and there is no difference in short-term neonatal outcomes between the groups. More research is needed that how does affect maternal and neonatal outcomes difference in oxidative stress according to mode of delivery in normal pregnancy.
In conclusion, parameters indicating oxidative stress in cord blood were higher in patients delivering with planned CS as compared to those with a vaginal birth. Total antioxidant capacity was higher in umbilical cord blood from patients with a planned CS as compared to a VD. Therefore, we propose that both the mother and neonate are exposed to higher oxidative stress.
References
1. Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 1994;344:721-724. PMID: 7915779.


2. Fridovich I. Oxygen toxicity: a radical explanation. J Exp Biol 1998;201(Pt 8):1203-1209. PMID: 9510531.



3. Yaacobi N, Ohel G, Hochman A. Reactive oxygen species in the process of labor. Arch Gynecol Obstet 1999;263:23-24. PMID: 10728623.


4. Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol (Oxf) 2002;57:609-613. PMID: 12390334.


5. Walsh SW, Wang Y. Secretion of lipid peroxides by the human placenta. Am J Obstet Gynecol 1993;169:1462-1466. PMID: 8267047.


6. Clerici G, Slavescu C, Fiengo S, Kanninen TT, Romanelli M, Biondi R, et al. Oxidative stress in pathological pregnancies. J Obstet Gynaecol 2012;32:124-127. PMID: 22296419.


7. Mondal N, Bhat BV, Banupriya C, Koner BC. Oxidative stress in perinatal asphyxia in relation to outcome. Indian J Pediatr 2010;77:515-517. PMID: 20401708.


8. Akisu M, Kullahcioglu Girgin F, Baka M, Husseyinov A, Kultursay N. The role of recombinant human erythropoietin in lipid peroxidation and platelet-activating factor generation in a rat model of necrotizing enterocolitis. Eur J Pediatr Surg 2001;11:167-172. PMID: 11475112.


9. Yi─¤it S, Yurdakok M, Kilinc K, Oran O, Erdem G, Tekinalp G. Serum malondialdehyde concentration as a measure of oxygen free radical damage in preterm infants. Turk J Pediatr 1998;40:177-183. PMID: 9677722.

10. Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res 2009;66:121-127. PMID: 19390491.


11. Siddiqui IA, Jaleel A, Tamimi W, Al Kadri HM. Role of oxidative stress in the pathogenesis of preeclampsia. Arch Gynecol Obstet 2010;282:469-474. PMID: 20549510.


12. Aydemir O, Akar M, Uras N, Eras Z, Erdeve O, Oguz SS, et al. Total antioxidant capacity and total oxidant status in perinatal asphyxia in relation to neurological outcome. Neuropediatrics 2011;42:222-226. PMID: 22144010.


13. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-358. PMID: 36810.


14. Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J Biol Chem 1987;262:5488-5491. PMID: 3571220.


15. Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med 1993;14:303-311. PMID: 8458588.


16. Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, LLeonart ME. Oxidative stress and cancer: an overview. Ageing Res Rev 2013;12:376-390. PMID: 23123177.


17. Giles GI, Tasker KM, Jacob C. Hypothesis: the role of reactive sulfur species in oxidative stress. Free Radic Biol Med 2001;31:1279-1283. PMID: 11705707.


18. Simic MG, Bergtold DS, Karam LR. Generation of oxy radicals in biosystems. Mutat Res 1989;214:3-12. PMID: 2671698.


19. Perry G, Friedland RP, Petot GJ, Nunomura A, Castellani RJ, Kubat Z, et al. Alzheimer as a disease of metabolic demand:benefits of physical and brain exercise. In: Radak Z, editors. Exercise and diseases: prevention through training. Oxford: Meyer & Meyer Sport; 2005. p. 7-16.
20. Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology 2005;6:71-75. PMID: 15834665.


21. Yu BP, Chung HY. Adaptive mechanisms to oxidative stress during aging. Mech Ageing Dev 2006;127:436-443. PMID: 16497363.


22. Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci 2006;7:278-294. PMID: 16552414.



23. Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem 2005;16:129-137. PMID: 15741046.


24. Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol 2004;122:369-382. PMID: 15248072.


25. Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig 2004;11:342-352.


26. Haga P, Lunde G. Selenium and vitamin E in cord blood from preterm and full term infants. Acta Paediatr Scand 1978;67:735-739. PMID: 213931.


27. Gopinathan V, Miller NJ, Milner AD, Rice-Evans CA. Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett 1994;349:197-200. PMID: 8050565.


28. Hara K, Yamashita S, Fujisawa A, Ishiwa S, Ogawa T, Yamamoto Y. Oxidative stress in newborn infants with and without asphyxia as measured by plasma antioxidants and free fatty acids. Biochem Biophys Res Commun 1999;257:244-248. PMID: 10092541.


29. Aydemir C, Dilli D, Uras N, Ulu HO, Oguz SS, Erdeve O, et al. Total oxidant status and oxidative stress are increased in infants with necrotizing enterocolitis. J Pediatr Surg 2011;46:2096-2100. PMID: 22075338.


30. Russell AP, Hesselink MK, Lo SK, Schrauwen P. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. FASEB J 2005;19:986-988. PMID: 15814608.


31. Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev 2008;7:34-42. PMID: 17869589.


32. Mutlu B, Aksoy N, Cakir H, Celik H, Erel O. The effects of the mode of delivery on oxidative-antioxidative balance. J Matern Fetal Neonatal Med 2011;24:1367-1370. PMID: 21247235.


33. Khaw KS, Wang CC, Ngan Kee WD, Pang CP, Rogers MS. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth 2002;88:18-23. PMID: 11883375.


34. Khaw KS, Wang CC, Ngan Kee WD, Tam WH, Ng FF, Critchley LA, et al. Supplementary oxygen for emergency Caesarean section under regional anaesthesia. Br J Anaesth 2009;102:90-96. PMID: 19011261.


-
METRICS

-
- 25 Crossref
- 3,114 View
- 35 Download
- Related articles in Obstet Gynecol Sci