Surgical treatment for apparent early stage endometrial cancer
Article information
Abstract
Most experts would agree that the standard surgical treatment for endometrial cancer includes a hysterectomy and bilateral salpingo-oophorectomy; however, the benefit of full surgical staging with lymph node dissection in patients with apparent early stage disease remains a topic of debate. Recent prospective data and advances in laparoscopic techniques have transformed this disease into one that can be successfully managed with minimally invasive surgery. This review will discuss the current surgical management of apparent early stage endometrial cancer and some of the new techniques that are being incorporated.
Introduction
Cancer of the uterine corpus consistently ranks as the most common gynecologic malignancy in the United States. In 2013, there will be an estimated 49,560 new cases diagnosed in the United States [1]. Despite the high incidence, the estimated number of deaths is relatively low: 8,190 [1]. The majority of cases will be indentified at an early stage, when often they can be cured with surgery alone. This is due to the fact that most patients with this disease develop an early warning sign, abnormal vaginal bleeding.
Most experts would agree that the standard surgical treatment for endometrial cancer includes a hysterectomy and bilateral salpingo-oophorectomy (BSO); however, the benefit of full surgical staging with lymph node dissection in patients with apparent early stage disease remains a topic of debate. Recent prospective data and advances in laparoscopic techniques have transformed this disease into one that can be successfully managed with minimally invasive surgery in the majority of cases. This review will discuss the surgical management of apparently early stage endometrial cancer and some of the new techniques that are being incorporated.
Definition of early stage disease (clinical stage I/II) and prognostic factors
In 1971, the International Federation of Gynecology and Obstetrics (FIGO) instituted clinical staging guidelines for endometrial cancer [2]. Disease limited to the uterine corpus is designated stage I disease, with a threshold of 8 cm for uterine corpus length used as the division between IA (≤8 cm) and IB (>8 cm) disease. However, several surgical-pathologic studies of endometrial cancer have identified prognostic factors for disease recurrence and progression, including histology, depth of myometrial invasion, tumor grade, cervical extension, vascular space invasion, and extrauterine metastasis [3,4]. Recognizing the need for a surgical assessment of disease, FIGO introduced a surgical-pathologic staging system for endometrial cancer in 1988 [5]. This procedure was initially carried out through an abdominal incision and included exploration of the peritoneal cavity, peritoneal washings, biopsies of any suspicious lesions, total abdominal hysterectomy (TAH), BSO, and retroperitoneal pelvic and para-aortic lymph node sampling. In the Gynecologic Oncology Group (GOG) 33 study, Creasman et al. [4] reported that 22% of patients with clinical stage I disease were upstaged after surgical staging due to extrauterine disease. Such a difference is associated with important prognostic and therapeutic implications, This led to the revision of the staging system for uterine cancer to be based on the surgical findings. The previously described clinical staging system can still be used when surgery is not feasible.
In 2009, the FIGO revised the staging system for endometrial cancer. Patients with stage I disease have disease limited to the uterus, with stage further divided into subgroups A and B based on invasion to less than one-half the myometrium, and invasion to more than one-half the myometrium, respectively [6]. The depth of myometrial invasion is an important prognostic factor with 5-year survival rates decreasing from 94% in patients with tumor confined to the endometrium to 59% for myometrial invasion to the outer third. Only 1% of patients with disease limited to the endometrium have pelvic and/or para-aortic lymph node involvement. In comparison, patients with deep one-third invasion had 25% pelvic lymph node and 17% para-aortic lymph node metastases rates. The grade of the tumor also constitutes an important prognostic factor, with lymph node metastases increasing as grade increases (pelvic lymph node metastasis: grade 1, 3%; grade 2, 9%; and grade 3, 18%) [4].
Despite the sub-clinical rate of extra-uterine disease, the majority of patients will have organ confined disease. Patients with disease confined to the endometrium have very small risk of lymphatic metastasis. This has led many to advocate limiting surgical staging to patients with myometrial invasion or higher-grade histology. These factors can be difficult to predict prior to or during surgery. While authors have reported rates of 85% to 91% for accurate gross intraoperative assessment of myometrial invasion, Goff and Rice [7] also reported on decreasing accuracy of depth of invasion with increasing grade (87.3% for grade 1, 64.9% for grade 2, and 30.8% for grade 3 lesions). Others have also reported similar inaccuracies of frozen section [7-10]. In addition to this, histologic grade determined by preoperative endometrial sampling (endometrial biopsy [EMB] or dilation and curettage [D&C]) have been associated with high rates of inaccuracy when compared with final hysterectomy specimen grade in some series (20%-30% with D&C, 42% by EMB) [11-14]. These diagnostic inaccuracies have led some to advocate that all patients with early-stage endometrial cancer should undergo surgical staging. Specifically, routine lymphadenectomy may result in an annual cost saving of $123.3 million based on a decision analysis model incorporating the costs of surgery as well as adjuvant therapy [15].
Certainly, surgical treatment is individualized to patient needs, and co-morbid conditions may limit the extent of surgical treatment. In certain patients, a vaginal hysterectomy alone may be used to resect the primary site of disease while placing the patient at decreased risk of morbidity [16].
1. Laparoscopic surgery
While traditional surgical staging for endometrial cancer was performed through a large incision, there have been many advances in minimally invasive surgical techniques over the past 3 decades that have allowed the oncologist to employ it more readily in the management of gynecologic cancer. Childers et al. [17] first described the use of laparoscopy to manage patients with endometrial cancer. Since that time, there have been multiple studies reporting on the use of a combined laparoscopic and vaginal approach to perform endometrial cancer staging, including a complete assessment of peritoneal surfaces and the retroperitoneum [18-21].
Potential benefits of laparoscopy are many. There have been several retrospective studies that have demonstrated decreased postoperative morbidity, pain, recovery time, operative time, and complications as well as increased patient satisfaction and quality of life associated with a laparoscopic approach. Gemignani et al. [22] performed a retrospective review of 320 patients with early-stage endometrial cancer treated by laparoscopically assisted vaginal hysterectomy (LAVH) or TAH. They found that LAVH was associated with a decrease in hospital stay, fewer complications, and lower overall hospital charges. Several other authors have reported similar findings. Cost comparisons have had mixed results, with similar or even increased cost associated with the laparoscopic approach [18,23-25].
While benefits such as decreased pain and recovery time are historically well described in patients with benign disease, the feasibility and oncologic outcomes were heavily scrutinized. General feasibility of minimally invasive approaches for the management of endometrial cancer were described in several early studies. Eltabbakh et al. [18] reported the results of a prospective series of 86 women with clinical stage I disease who underwent laparoscopic staging. Pelvic and/or para-aortic lymph node dissection was performed based on risk assessment due to myometrial invasion, tumor grade, or high-risk histologic type, such as papillary serous or clear cell tumors (pelvic lymph node dissection in patients with myometrial invasion, grade >1, or high-risk histology; para-aortic lymph node dissection in patients with grade 3 disease and myometrial invasion, invasion of greater than half depth, or high-risk histology). Mean operating time was 190 minutes; mean estimated blood loss (EBL) was 278.3 mL and conversion to laparotomy occurred in 5 (5.8%) of 86 patients. When compared with their own historical controls of patients undergoing open staging, they found an increase in mean pelvic lymph nodes harvested (10.8 with laparoscopy vs 4.9 with open surgery), while para-aortic lymph node count was not significantly different (2.7 with laparoscopy vs open 4.2 with open surgery). Laparoscopy was associated with longer surgical time, no significant difference in major complications, and decreases in EBL, pain medication, and hospitalization.
Kohler et al. [20] described the adequacy of lymphadenectomy in an analysis of 650 laparoscopic pelvic and/or para-aortic transperitoneal lymphadenectomies in patients with gynecologic malignancies. Of the 112 patients with endometrial cancer, 66 presented for primary surgery with LAVH, BSO, and complete pelvic and para-aortic lymph node dissection. A mean of 26.7 lymph nodes (pelvic, 15.4; para-aortic, 9.6) were harvested, with respective mean durations of 56 and 63 minutes for pelvic and para-aortic lymph node dissections, respectively.
Several retrospective studies have examined the outcomes and survival of laparoscopic surgery for endometrial cancer and found comparable results. Eltabbakh [26] found similar 2- and 5-year overall survival rates with no difference in sites of recurrence in 100 patients undergoing laparoscopy compared with patients undergoing laparotomy. In a review of 45 patients with stage I disease with a mean follow-up of 6.4 years, Magrina and Weaver [27] found a 5-year recurrence rate of 4.9% and a 5-year cause-specific survival rate of 94.7%-rates similar to those achieved with laparotomy.
Smaller prospective randomized controlled trials have also compared the combined laparoscopic and vaginal approach to abdominal approach in the management of endometrial cancer. Malur et al. [23] compared 37 patients treated by a laparoscopically assisted approach with 33 patients treated by laparotomy. Pelvic and aortic lymph node dissections were performed, except in patients with well-differentiated tumors that invaded to less than the inner third of the myometrium. While there was no difference in mean number of lymph nodes and mean operation time, patients who underwent laparoscopic staging had less blood loss, fewer transfusions, and a shorter hospital stay. With a mean follow-up of 16.5 months (range, 2-43 months) and 21.6 months (range, 2-48 months) for the laparoscopic and laparotomy groups, respectively, the recurrence-free and overall survival rates were not significantly different (97.3% vs. 93.3% and 83.9% vs. 90.9%, respectively). Fram [24] performed a randomized study on 61 clinical stage I patients triaged to laparoscopic versus open staging. Laparoscopy was associated with a significant decrease in EBL (145 vs. 501 mL) and hospitalization (2.3 vs. 5.5 days); there was not much difference in the number of lymph nodes removed (21.3 vs. 21.9).
Several factors have been considered in the assessment of appropriate candidates for a laparoscopic approach. Obesity ranks as one of the highest risk factor for the development of endometrial cancer and many patients will present with a high body mass index (BMI). Although technically more demanding, obesity does not translate into an absolute contraindication to performing a laparoscopic staging procedure. The feasibility of laparoscopic pelvic and para-aortic lymphadenectomy in the obese patient was described by Scribner et al. [28]. In 55 patients, laparoscopic staging was completed in 82% of patients with a quetelet index (QI) <35 compared with only 44% in patients with a QI ≥35 (P=0.004). Despite this difference, the authors concluded that obesity is not an absolute contraindication to laparoscopic staging. Kohler et al. [20] found no significant difference in lymph node yield according to BMI in patients undergoing laparoscopic para-aortic lymphadenectomy; however, they reported a significant increase in duration of right-sided para-aortic lymph node dissections in patients with a BMI >30. They attribute this to increased difficulty with exposure in the para-aortic region- a problem they did not encounter in the pelvis. Scribner et al. [29] also examined the factor of age in a group of endometrial cancer patients who were 65 years or older. Although the operating time and transfusion rate were significantly higher in the patients managed by laparoscopy compared to laparotomy, laparoscopy was associated with decreased hospital stay, incidence of ileus, and infectious complications, with comparable blood loss and lymph node counts removed.
Laparoscopic surgery should be considered as a modality that can be used for the management of endometrial cancer. Like any other medical treatment, it too has its limitations. Anatomic barriers, such as large uteri that require morcellation, are contraindications to using laparoscopic surgery if endometrial cancer is present.
The GOG conducted a large randomized trial to compare laparoscopy and laparotomy for the comprehensive surgical staging of uterine cancer [30]. Named the "LAP-2 trial," 2,616 patients were randomly assigned to surgical staging of uterine cancer by either laparotomy (920 patients) or laparoscopy (1,696 patients). There was a 26% rate of conversion to laparotomy in the patients who were randomized to laparoscopy, primarily due to poor visibility. Patients in the laparoscopic arm did not have pelvic and aortic nodes removed in a higher percentage of patients when compared to the laparotomy group (8% vs. 4%, P<0.001). There was no difference in the overall detection of advanced stage disease in the 2 groups. Despite having a longer operative time, the LAP-2 trial confirmed that hospital stay was shorter and there were fewer postoperative adverse events in the laparoscopic group. Laparoscopic surgery was also associated with an improved quality of life [31]. In the follow-up study reporting on disease outcomes, the study was found to fall short of the protocol-specified definition of noninferiority. The authors pointed out that the actual recurrence rates were substantially lower than anticipated; the 3-year recurrence rate was 11.4% with laparoscopy and 10.2% with laparotomy. The estimated 5-year overall survival rate was 89.8% in both arms, leading the authors to conclude that laparoscopy is a reasonable method to surgically treat patients with early stage uterine cancer [32].
2. Robotic surgery
Although there have been many advances in the field of minimally invasive surgery, the introduction of a computer-based robotic platform will certainly be remembered as one of the most significant of recent times. Despite results from the GOG LAP-2 study, the use of laparoscopy for the management of uterine cancer has not been uniformly adopted. Reasons for not performing laparoscopy included weight, history of prior surgeries, medical co-morbidities, or technical difficulties [33]. In 2005, only 14% of hysterectomies performed in the United States were done with laparoscopy [34]. However, that year a computer-based robotic platform was approved by the Food Drug Administration for use in gynecologic surgery. Early reports demonstrated that robotic hysterectomy and lymphadenectomy could be accomplished with less morbidity in heavier patients when compared to laparoscopy [35]. The use of a robotic platform also resulted in a shorter operative time, length of stay, a lower transfusion rate, and less frequent conversion to laparotomy [36].
Recently, Leitao et al. [37] reported on a modern day series of 752 patients with newly diagnosed uterine cancer who underwent initial surgical management at a large cancer center from 2007 to 2010. One hundred four (14%), 302 (40%), and 347 (46%) patients underwent planned laparotomy, laparoscopy, and robotic surgery, respectively. When comparing the preoperative characteristics of the laparoscopic and robotic cohorts, there was a high proportion of morbidly obese patients in the robotic group, 15% vs. 10%, P=0.049. The median total operating room time was higher for the robotic group versus the laparoscopic group (213 vs. 184 minutes, P<0.001). However, after accounting for a 40 case learning curve, these operating room times were similar. Median estimated blood loss was 50 mL for the robotic group compared to 100 mL for the laparoscopic (P<0.001). Median pelvic node counts were 13 (range, 3-34) and 15 (range, 3-48) for the robotic and laparoscopic groups respectively (P=0.03). Median postoperative stay was 1 day (range, 0-5 days) for the robotic group compared to 2 days (range, 1-15 days) for the laparoscopic group (P<0.001). Interestingly, the increased use of robotic surgery from 8% to 64% of the newly diagnosed uterine cancer population coincided with a decrease in the use of planned laparotomy from 24% to 9%. The authors concluded that the computer-assisted robotic platform could be efficiently introduced into the management of newly diagnosed endometrial cancer and may lead to a reduction in the rate of laparotomy. This decrease in laparotomy may also translate into cost savings [38].
Robotic surgery in this patient population has been shown to be associated with significantly lower postoperative pain and pain medication requirements, which may be another attractive patient benefit of this approach [37].
3. Surgical staging
The role of surgical staging in the management of endometrial cancer remains a debated topic. The question of whether pelvic and para-aortic lymphadenectomy is diagnostic or therapeutic remains unclear. Several retrospective studies have demonstrated a therapeutic benefit to lymph node dissection. Kilgore et al. [39] reported on a retrospective series of patients undergoing multisite lymph node sampling compared with a group of patient who underwent limited or no lymph node sampling. Patients were further separated into a low-risk group (disease confined to the uterus) and a high-risk group (disease outside the uterus) for additional comparisons. There was an improved survival in patients who underwent a multisite lymph node sampling compared with no node sampling. Survival was also improved in the low risk group compared with the high risk group, with and without radiation therapy and for poorly differentiated tumors when multisite lymph node sampling was performed. Other retrosepective studies have also supported the possible therapeutic benefit of selective lymphadenectomy in the management of patients with apparently early stage endometrial cancer [40].
Despite these retrospective data, there have been two randomized studies that have refuted the benefit of lymphadenectomy. In an Italian study of over 500 patients with stage I endometrial cancer, Panici et al. [41] demonstrated no difference between those patients who underwent lymphadenectomy and those who did not. Disease-free survival was 80% in the lymphadenectomy group compared to 82% in the no lymphadenectomy group. Overall survival rates of 90% and 86%, respectively, were also comparable. A second randomized trial from the United Kingdom also refuted the benefit of lymphadenectomy. Entitled "A Study in the Treatment of Endometrial Cancer (ASTEC)," the authors included 1,400 patients with disease confined to the uterus on preoperative assessment [42]. Patients were randomized undergo a pelvic lymphadenectomy or not. The hazard ratios for overall survival and recurrence-free survival were 10.4 and 1.25, respectively, in favor of the no lymphadenectomy group. Despite offering prospective evidence to support not performing a lymphadenectomy, these trials have received criticism for being performed in the low risk populations and being underpowered. Specifically, the ASTEC trial had baseline differences in the two arms and aortic lymphadenectomy was not required. Node status was entirely ignored in the decisions regarding postoperative therapy and many of the node-positive patients did not receive any postoperative therapy and only rarely received chemotherapy. The Italian study was criticized for a disproportionate use of adjuvant treatment in the no lymphadenectomy group and the 16% of patients randomized to lymphadenectomy actually had lymph nodes removed. Evaluation of over 12,000 patients from the Surveillance, Epidemiology and End Results (SEER) database found an improved 5-year disease specific survival with lymphadenectomy in stage IB grade 3 and higher patients [43]. With the thought that lymphadenectomy may be of benefit in the higher risk group, the GOG has proposed a prospective trial to evaluate the role of lymphadenectomy in high risk patients.
4. Sentinel node mapping
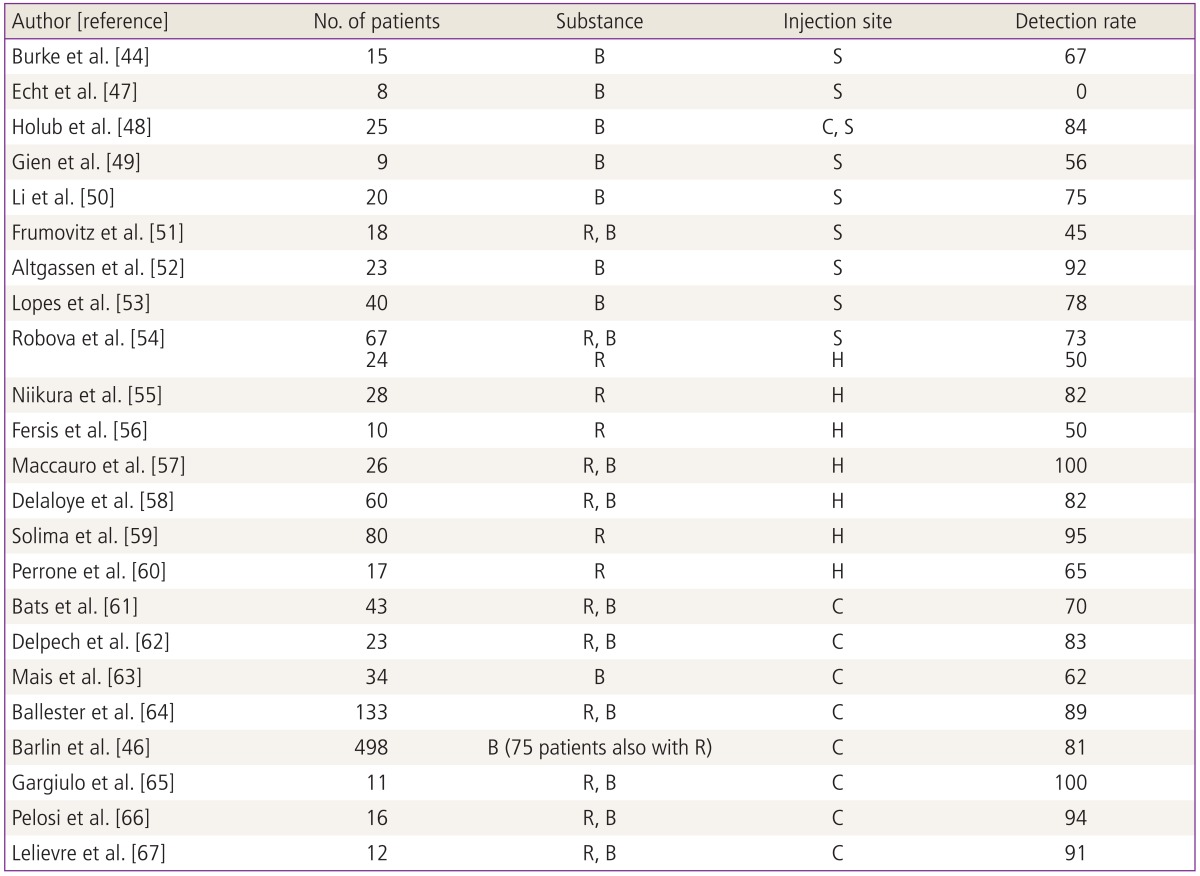
With the benefit of complete lymphadenectomy being in question, investigators have been studying the role of sentinel node mapping in the management of endometrial cancer (Table 1). The technique has been employed in the management of breast cancers and cutaneous melanoma, but its use in the management of gynecologic malignancies is still in its infancy. Sentinel node mapping is intended to minimize the potential morbidity of complete lymphadenectomy while providing an accurate assessment of the lymph nodes. It may ultimately be the ideal route for endometrial cancer surgical staging, lying between no evaluation of lymph node status and a full pelvic and para-aortic lymphadenectomy.
Sentinel node mapping for uterine cancer was first described by Burke et al. [44]. They reported on 15 patients who had sentinel node mapping followed by complete pelvic and para-aortic lymphadenectomy. They reported an overall sentinel node detection rate of 67%. There were 4 patients (27%) found to have nodal metastasis. Two of these patients had nodal metastasis marked by blue dye. One of the 4 had a positive non sentinel node, and the final patient had bulky nodes without any update of dye. Since this initial report, there have been multiple other reports of sentinel node mapping in patients with uterine cancer. Techniques have included cervical, subserosal, and hysteroscopic injection with detection rates ranging from 45% to 100%.
A recent French prospective study evaluated the role of sentinel node mapping in early stage endometrial cancer (Senti-Endo study) [45]. There were 133 patients enrolled at 9 centers. The authors employed a combination of patent blue dye and technetium colloid. Eight patients were excluded from the study, leaving 125 evaluable patients. There were 111 patients who had a sentinel node detected, for an overall detection rate of 89%. The sentinel node detection rate was 77% and 76% in the right and left pelvis, respectively. There were 34 (31%) of unilateral cases and 77 (69%) of bilateral cases. When considering the hemipelvis as a unit, there were no false negative cases and the sensitivity and negative predictive value were 100%. When analyzing the results per patient, there were three false negatives resulting in a sensitivity of 84% and negative predictive value of 97%. Of the three false negative cases, two had metastasis found in the hemipelvis contralateral to the sentinel node and one had a para-aortic metastasis. All patients had type 2 endometrial cancers. The authors concluded that sentinel node mapping could be a "trade-off" between systematic lymphadenectomy and no lymph node evaluation in patients with low or intermediate risk endometrial cancer.
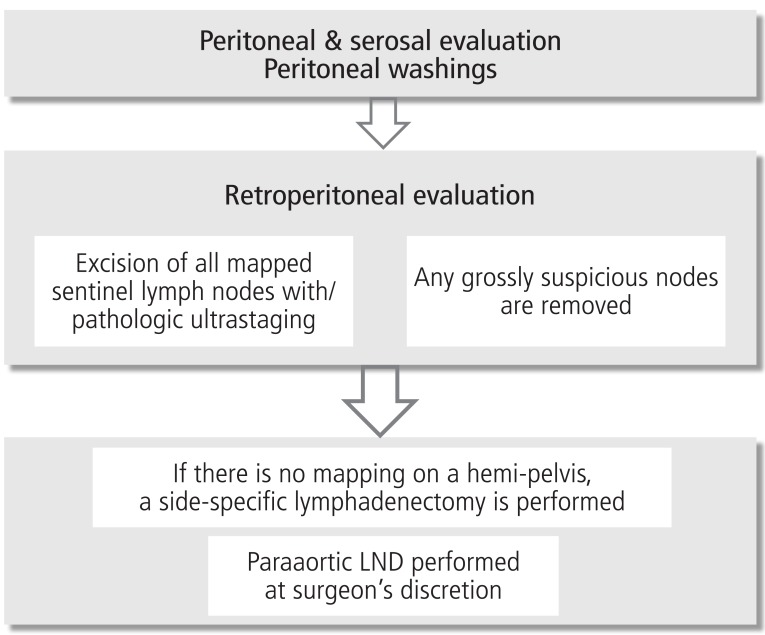
In an effort to minimize the false negative rate of sentinel node mapping, Barlin et al. [46] have reported on a sentinel node mapping algorithm (Fig. 1). The authors reported on 498 patients who underwent sentinel node mapping. The sentinel node correctly diagnosed 40 of 47 patients with nodal metastases who had at least one sentinel node mapped, resulting in a false negative rate of 15%. After applying the algorithm, the false negative rate for detecting nodal metastasis dropped to 2%. The authors proposed that the algorithm be employed to minimize the false negative rate reported with sentinel node mapping alone.
Conclusion
Technical advances have allowed surgery for apparent early stage endometrial cancer to incorporate a minimally invasive approach. Randomized studies have demonstrated that comparable oncologic outcomes can be obtained using laparoscopy compared to laparotomy while providing improved quality of life. The introduction of a computer-based robotic platform has provided an alternative to the technically demanding traditional laparoscopic surgery and may allow more patients with endometrial cancer to be treated with minimally invasive surgery. Additionally, incorporation of a sentinel node mapping algorithm appears promising and ultimately could help accurately guide treatment while minimizing morbidity. Additional larger studies are needed to confirm the utility of these promising techniques for the surgical management of apparent early stage endometrial cancer.
Notes
No potential conflict of interest relevant to this article was reported.