|
 |
- Search
| Obstet Gynecol Sci > Volume 56(2); 2013 > Article |
Abstract
Since the existence of cell-free fetal DNA (cff-DNA) in maternal circulation was discovered, it has been identified as a promising source of fetal genetic material in the development of reliable methods for non-invasive prenatal diagnosis (NIPD) of fetal trisomy 21 (T21). Currently, a prenatal diagnosis of fetal T21 is achieved through invasive techniques, such as chorionic villus sampling or amniocentesis. However, such invasive diagnostic tests are expensive, require expert technicians, and have a miscarriage risk approximately 1%. Therefore, NIPD using cff-DNA in the detection of fetal T21 is significant in prenatal care. Recently, the application of new techniques using single-molecular counting methods and the development of fetal-specific epigenetic markers has opened up new possibilities in the NIPD of fetal T21 using cff-DNA. These new technologies will facilitate safer, more sensitive and accurate prenatal tests in the near future. In this review, we investigate the recent methods for the NIPD of fetal T21 and discuss their implications in future clinical practice.
Prenatal testing is an integral component of obstetric practice, and is commonly performed in professional medical organizations. The primary aim of prenatal testing is the diagnosis of fetal aneuploidies, such as trisomy 21 (T21, Down syndrome), trisomy 18 (Edwards syndrome), and trisomy 13 (Patau syndrome), as well as aneuploidies related to the X and Y chromosomes [1]. Although the majority of fetuses with aneuploidy result in termination during the development of the fetus, T21 has the highest survival rate, which affects 1 in 800 births [2]. Therefore, the prenatal detection of T21 is considered the most common and important aspect of prenatal genetic testing.
Prenatal testing of T21 falls into 'screening' and 'diagnosis' category. Current prenatal screening tests have greatly improved by using a combination of maternal serum markers and fetal sonographic markers such as nuchal translucency [3-6]. The best performing screening tests are able to identify more than 90% of T21 cases, with a 5% rate of false positives. However, positive screening results require confirmation with diagnostic testing, such as amniocentesis or chorionic villus sampling (CVS). The accuracy of these diagnostic methods is estimated to be 98% to 99% [7]. However, both sampling procedures are invasive, and are associated with significant risks to the fetus and mother, including the potential loss of a healthy fetus [7,8]. For this reason invasive prenatal diagnosis tests are currently preformed only in high-risk pregnancies or in pregnancies with increased maternal age and/or family history of having a child with an inherited disease. Therefore, developing a reliable method for non-invasive prenatal diagnosis (NIPD) for fetal T21 is of critical importance in prenatal care.
To perform NIPD, a source of fetal genetic material that could be sampled without harm to the fetus would be needed. Since the 1970s, researchers have isolated intact fetal cells in maternal circulation [9]. However, fetal cells in maternal blood are rare in quantity and tend to remain in the mother's body for years [10]. Hence, this method is unsuitable for NIPD [11]. In 1997, Lo et al. [12] discovered the existence of cell-free fetal DNA (cff-DNA) in maternal circulation. Compared to fetal cells, cff-DNA is relatively more abundant in maternal blood and thus has been regarded as a promising new material for NIPD. It constitutes approximately 10% of the total DNA in maternal plasma and is rapidly cleared from maternal blood, within two hours of delivery [13,14]. Moreover, it has recently been found that the entire fetal genome, in the form of cff-DNA, is present in maternal blood [15]. Therefore, cff-DNA has become the focus of research for the development of NIPD.
Currently, the clinical potential of cff-DNA has been demonstrated. In particular, the determination of fetal sex and fetal Rhesus D status using cff-DNA is already applied as routine tests in Denmark, Sweden, and the Netherlands [16-18]. However, the application of cff-DNA for the NIPD of fetal T21 has been considered to be technically challenging. One aspect of the challenge relates to the presence of the large amount of maternal DNA which interferes with the analysis of the fetal DNA in maternal plasma [13]. Another challenge is related to the characteristics of the cff-DNA that pose a difficulty in determining the chromosome dosage of the fetus. Recently, various methods have been applied to overcome these challenges in the NIPD of fetal T21 using cff-DNA and promising results have been reported. In this review, we discuss the most recent technologies for the NIPD of fetal T21 using cff-DNA, and their use in clinical practice.
The need for reliable methods for NIPD of T21 has created a strong interest in the field of rapid and accurate single-molecule counting methods (e.g., digital polymerase chain reaction [PCR] and massively parallel DNA sequencing [MPS]), which could be used in routine clinical diagnosis in the form of automated platforms [19]. The single molecule counting techniques can detect fetal aneuploidy without the analysis of fetal-specific DNA in maternal plasma [20]. These methods are based on the detection of the extra copy of chromosome 21 to distinguish normal cases from T21 cases. For example, in cases where a woman is carrying a fetus with T21, the number of copies of chromosome 21 in the maternal blood is expected to be slightly higher in comparison with other autosomes. Currently, rapidly developing MPS technologies, which provide a vast amount of data across the entire genome, appear to be suitable for counting genome representation and determining the over-represented chromosomes 21 in the affected fetus. Moreover, these techniques can simultaneously detect all trisomies in a single step.
Digital PCR is a single molecule counting technique that allows the quantification of DNA by counting one molecule at a time. It has superior analytical precision compared with conventional PCR methods. Thus, it can precisely quantify small increments within the total (maternal+fetal) amount of DNA molecules derived from chromosome 21 for T21, when compared with euploid pregnancies. Lo et al. [21] reported on the application of digital PCR for the NIPD of T21. They used an approach called relative chromosome dosage where the amount of plasma DNA molecules from chromosome 21 was compared with that of a reference chromosome, that is, a chromosome expected to have a normal dosage in the fetus [21]. The relative chromosome dosage of chromosome 21 to the reference chromosome was elevated in maternal plasma of women with T21 fetus and the degree of increments was dependent on the fetal DNA concentration. However, in the application of digital PCR for the NIPD of fetal T21, the analytical platform of digital PCR needs to be quantitatively more precise to reliably determine the small expected increment. Quantitative precision can be improved by increasing the number of PCR analyses performed. A previous study has shown that the accurate detection of fetal T21 in a maternal plasma sample containing 25% fetal DNA requires approximately 8,000 digital PCRs [21]. Therefore, the clinical setting for the NIPD of fetal T21 using digital PCR may require the use of automated platforms.
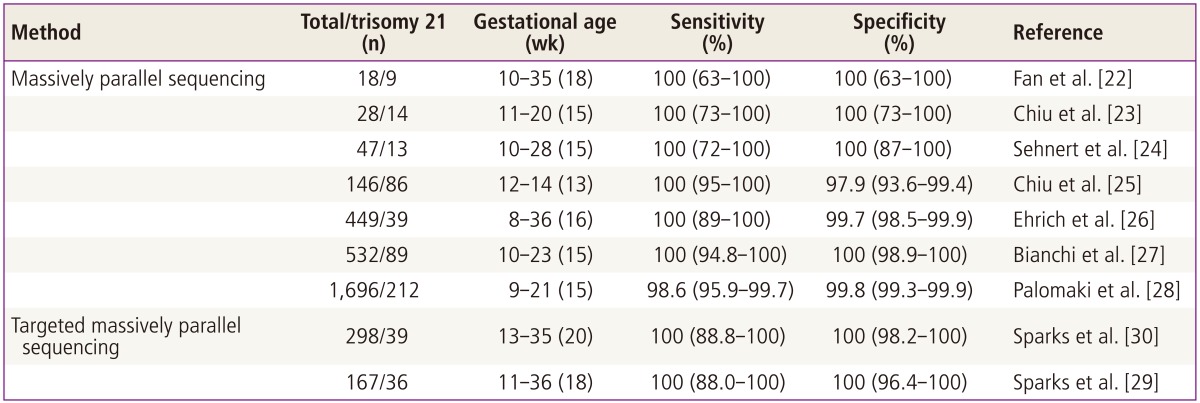
New next-generation DNA sequencing (NGS) technologies permit the simultaneous sequencing of extremely large quantities of DNA molecules. NGS produces millions or billions of short sequence reads per instrument run. NGS of cff-DNA from maternal blood has enormous potential, not only for increasing our understanding of the causes of prenatal genetic disorders in the fetus but also for designing non-invasive clinical diagnostic tests [15]. The possibility of using NGS to detect non-invasive fetal trisomy from maternal blood has been demonstrated [22-24], and this finding has been confirmed in other recent studies (Table 1) [25-30]. An alternative approach to sequencing whole genomes for the non-invasive detection of fetal abnormalities is to enrich only interest regions prior to sequencing [29-31]. Moreover, NGS technologies show remarkable potential for detecting the most common aneuploidies, including T21, T18, and T13. Currently, these discoveries have been translated into clinical tests, resulting in major benefits for NIPD.
Generally, the NIPD of fetal T21 using NGS is done through the following process. First, a short region at one end of each DNA molecule of maternal plasma is sequenced using synthesis technology and mapped against the reference human genome to determine the chromosomal origin of each sequence. Next, the density of the sequenced tags from the chromosome 21 of interest from a T21 fetus is compared with cases of trisomy and euploid pregnancies. Consequently, NGS can clearly identify samples from women carrying aneuploid fetuses by comparing them with samples taken from women with known euploid fetuses. Previous studies demonstrated that NGS was highly accurate in the direct detection of fetal T21 from maternal plasma (Table 1) [22-30]. The accuracy of NGS for the NIPD of T21 has already been validated by large-scale clinical studies. However, sequence information of NGS is obtained for the various chromosomes proportional to their sizes.
Therefore, chromosome 21, being the smallest autosome, would only be represented by a relatively small percentage of the sequence reads. As a result, the throughput of NGS for NIPD of fetal T21 is too low. To overcome the limitations of NGS, several targeted sequencing approaches were developed based on the a priori selection of DNA regions for analysis. Compared to sequencing and counting all reads from chromosomes, limiting the number of DNA regions greatly reduces the effort required to assess the dosage of a chromosome. Moreover, the careful selection of the DNA regions to quantify can potentially reduce the confounding variation in the number of reads per locus by taking into account only the loci with similar properties (e.g., GC content or the number of repeats of a particular sequence in the genome) [29,30]. Sparks et al. [30] described a method for detecting chromosome aneuploidy using NGS combined with an amplificationbased enrichment assay. The assay is comprised of three oligos per analyzed locus. Of the 298 samples, including 39 trisomy 21 samples and seven trisomy 18 samples, all aneuploidy samples were correctly distinguished from the controls, and as such the authors concluded the assay to have 100% sensitivity and specificity. The level of sequencing, covering only 420,000 reads per sample, was nevertheless sufficient to detect trisomy 21 and trisomy 18 reliably (z statistics exceeded 3.6 in all samples). This level corresponds to a <5% of the level required by non-targeted approaches. Moreover, this method enables multiplexing (96 samples were processed simultaneously), thus greatly reducing the cost of the analysis. The recent single nucleotide polymorphism (SNP)-based targeted NGS method was developed for the NIPD of fetal aneuploidies [29,30] and seems to be highly efficient. The key feature of this method is that it takes the mixture of maternal and fetal DNA obtained from blood plasma into account, separately from the DNA from one or both parents. Along with T21, T18, T13, and sex chromosome aneuploidies (e.g., X0, XXY, XXX, XYY) can also be detected, which is an important advantage of this method in light of the high occurrence of these abnormalities. A clinical trial of the prenatal non-invasive aneuploidy testing using SNPs, supported by the National Institutes of Health, is currently underway [32]. As it is SNP-based, the method may need to be tested on patients from different populations. Nevertheless, as targeted DNA sequencing can be performed on a sequencing machine with a lower price per run and lower throughput (e.g., PGM, Ion Torrent [Life Technologies, San Francisco, CA, USA], or MiSeq [Illumina Inc., San Diego, CA, USA]), these methods are preferred, especially for average-sized clinics.
The major challenge for the development of NIPD using cff-DNA is that cff-DNA only constitutes around 10% of the total DNA in the maternal circulation [13]. To differentiate the fetal-derived sequences from that of the mother, the most intuitive targets for the detection of fetal DNA were based on absolute discriminative genetic markers, such as Y-chromosome-specific loci or paternally-inherited polymorphic loci that are either absent or different in the maternal genome [33-35]. However, these types of fetal markers were associated with certain limitations in practice. Firstly, diagnostic tests developed based on Y-specific targets could only be applied to pregnancies involving male fetuses. Secondly, the detection of a paternally inherited polymorphism requires prior knowledge of the polymorphic status of the parents, and could only apply to a subset of individuals who possessed that particular polymorphism. Therefore, it would be desirable to develop a type of marker that allows for a confident differentiation of the fetus from the mother, and yet is independent of the gender or polymorphic status of the fetuses. Recently, epigenetic modifications as fetal-specific signatures to detect cff-DNA from circulating maternal DNA have been investigated.
Epigenetic modifications refer to inheritable molecular processes that affect gene expression without changing the DNA sequence or content, and the most widely studied epigenetic process is DNA methylation. The possibility of DNA methylation as a non-invasive biomarker was first demonstrated in the plasma of patients with cancer [36-38]. Soon after such discoveries, various attempts have been made to identify fetal-specific epigenetic markers based on differential methylation patterns between the fetus and the mother [39-41]. Fetal-specific methylation pattern is divided to parent origin-specific methylation pattern and placenta specific methylation pattern.
First, parent origin-specific methylation pattern is based on genomic imprinting in humans [42,43]. Fetal epigenetic markers are developed with an imprinted region, in which the DNA methylation patterns are inherited in a parent origin-specific manner [44]. For example, if a pregnant woman has inherited the methylated copy of an imprinted region from her father, an imprinted region in her fetus would become unmethylated because she passed. The methylation status of this region is distinguishable between the fetus and the mother in an allele-specific manner. In 2002, the imprinted region between the IGF2 and H19 genes was investigated to detect fetal-specific methylation from maternal plasma [39] and was confirmed by genotyping a biallelic polymorphism within the differentially methylated regions [39]. However, this method would be relatively complicated to use as a routine fetal marker, because this marker was based on an imprinted locus. Next, placenta specific methylation pattern is based on the human placenta with a specific DNA methylation pattern that is different with somatic tissues [45-47]. The majority of cff-DNA in the maternal plasma was derived from the placenta, while the maternal cell free DNA in the maternal plasma was predominantly derived from the maternal hematopoietic cells [48-50]. Therefore, genomic regions that are differentially methylated between the placenta and the maternal blood cells have been considered as fetal-specific epigenetic makers in maternal plasma. In 2005, a region on the maspin (SERPINB5) gene promoter was firstly found to be hypomethylated in the placenta, while hypermethylated in the maternal blood cells [40], and the hypomethylated sequences of the SERPINB5 gene were detectable in maternal plasma throughout the course of pregnancy, and its level dropped significantly after delivery. Therefore, this was reported as the first universal fetal marker that can be used in all pregnancies, regardless of fetal gender and genotype. After this discovery, various attempts were made to identify a number of genomic regions that are differentially methylated between the placental tissue and the maternal peripheral blood cells according to the principle of NIPD. This feature allows for the development of a single, simple test to determine the presence of cff-DNA in the maternal plasma with greater simplicity and coverage. The approaches used for the detection of these markers are variable, depending on whether the placental-derived sequences are hypermethylated or hypomethylated compared with the maternal blood cells.
To detect fetal epigenetic markers in maternal plasma, the first step is to differentiate methylated and unmethylated sequences. Various methods, such as a bisulfite modification of the template DNA, differential cleavage by restriction enzymes and antibody-mediated enrichment of methylated fragments by methylated DNA immunoprecipitation (MeDIP), are applied. The next step is to quantify a fetal-specific methylation pattern. In general, PCR-based methods, such as quantitative methylation-specific PCR and quantitative real-time PCR, are used.
Briefly, the process of bisulfite conversion changes unmethylated cytosine residues into uracil, leaving methylated cytosine unchanged [51]. The bisulfite-converted DNA is differentially amplified by PCR-based methods, depending on the methylation status of the regions where the primers bind [52]. However, bisulfite DNA conversion results in the degradation of >90% of the template DNA [53]. Therefore, this technique is undesirable for the detection of cff-DNA, which is present at a lower abundance in maternal plasma, particularly during early gestation. Methylation sensitive restriction enzymes, such as BstU I or Hpa II, can also be distinguished to differentiate between methylation patterns in DNA sequences. These restriction enzymes sensitively digest ummethylated cytosine bases in their recognition sequence, such as CGCG or CCGG. To quantify cff-DNA in maternal blood using methylation-sensitive restriction enzymes, cell-free maternal DNA should be unmethylated. This unmethylated maternal DNA is removed in cell-free total plasma DNA by the treatment of such enzymes, and then can be quantified the digestion-resistant (methylated) cff-DNA by quantitative methods, including real-time PCR or digital PCR [41,54]. Compared with bisulfite conversion, this digestion-based method introduces less damage to the plasma DNA. However, the enzyme cleavage effectiveness, depending on the duration of digestion or the amount of enzymes used, can affect the quantification of cff-DNA [55]. Recently, MeDIP, which captures DNA containing methylcytosine, has been applied to quantify cff-DNA. This method can capture only methylated DNA fragments using a monoclonal antibody specific for methylcytosine and provides up to a 90-fold enrichment of methylated DNA. Generally, the unmethylated or methylated DNA sequences can be quantitatively measured by a methylation-specific PCR (MSP) using a fluorescence probe. The copy number is calculated directly from the amplification curves of the fluorescence signal by a series of calibration standards. This method has been widely used to identify methylation patterns of cff-DNA in maternal plasma [56,57] and applied to develop effective epigenetic tests for the NIPD of fetal T21.
Analysis of differences in the DNA methylation patterns between the maternal and fetal circulating DNA molecules has been proposed as an alternative strategy to the analysis of cff-DNA sequences in the NIPD of fetal T21. Such epigenetic markers could be useful either via the analysis of the epigenetic allelic ratios or directly compared with a placenta-derived DNA methylation marker on a reference chromosome.
The fetal-specific epigenetic markers require:1) the detection of a number of DNA sequences that are differentially methylated between maternal and fetal DNA and 2) quantification of these fetal-specific DNA sequences by methods such as quantitative MSP or quantitative real-time PCR. Previous studies described that PDE9A on 21q22.3, which were hypomethylated in the placental tissues while completely methylated in the maternal peripheral blood cells, can be used for the NIPD of T21 [58,59]. The putative promoter regions of HLCS on 21q22.13, which are hypermethylated in the placental tissue compared with the maternal blood cells, are also applied for the NIPD of fetal T21 and have reported promising results [60]. Theoretically, the allelic ratio of a fetal-specific epigenetic marker may present equal signal intensity for unaffected fetuses and an increased signal intensity of chromosome 21 for T21 fetuses. Using this approach, fetal T21 can be detected non-invasive even during the first trimester [42,60]. The enrichment of sequences which are specifically methylated in the placenta and/or the analysis of multiple informative markers on the chromosome 21 have been applied to detect fetal T21 with high sensitivity and specificity. Recently, various methylation-specific techniques, such as antibody-mediated enrichment of methylated fragments by MeDIP and differential amplification of methylated fragments via Hpa II tiny fragment enrichment by ligation-mediated PCR (HELP), were used for the NIPD of fetal T21 using fetal epigenetic markers [61-64]. The correct diagnosis in the NIPD for fetal T21 using fetal epigenetic markers is based on the ratio of a subset of fetal-specific methylated regions located on chromosome 21 compared with normal cases. This new platform is calculated with further statistical analysis of multiple markers and has exhibited excellent clinical performance (both the sensitivity and specificity were 100%) [65]. This methodology seems to be easily reproducible and can be readily performed by equipment currently present in most diagnostic laboratories without sophisticated analytical platforms. Moreover, this approach can be simultaneously detected in all known aneuploidies, if regions exist where the fetal DNA is hypermethylated compared to the maternal peripheral blood DNA are provided. Therefore, this technique seems to have the right properties to become a NIPD technique for T21 and would provide a cost-effective alternative. However, such an approach is limited in the practical applicability of NIPD for fetal T21 because of the low number of copies of cff-DNA in maternal blood and the variability in the levels of DNA methylation of individual fetal-derived epigenetic markers can affect the results and its clinical value remains to be proven in large-scale clinical studies.
The development of an NIPD technique for fetal T21 that would provide true genetic information without carrying risk for the progress of the pregnancy will continue to be an actively researched area in prenatal diagnosis. Trials performed so far highlight the medical and commercial potential of NIPD, but the proposed techniques warrant further validation in clinical practice. Throughout the last decade, considerable achievement has been made regarding the technical possibilities for the NIPD of T21. In the previous years, male-specific signals or paternally inherited polymorphisms have been proposed as targeted fetal DNA markers, but research interest has now evolved to the detection of fetal-specific patterns or epigenetic signatures with a unique methylation pattern that will allow the application of NIPD in all pregnancies. In parallel, novel sequencing methods with high diagnostic accuracy have already been applied in the clinical setting as an effective breakthrough for the NIPD using cff-DNA. Yet, population-based, double-blind, large-scale clinical trials are required to verify the diagnostic potential of these methods and their cost-effectiveness compared with the conventional screening tests before their introduction into the clinical practice of fetal medicine. In particular, the fact that NIPD using cff-DNA requires a small sample of maternal blood may create numerous ethical, social and legal implications, owing to the ease with which the test can be performed. Therefore, the use of this method should be carefully considered in clinical situations. Nevertheless, in the near future, the NIPD of fetal T21 using cff-DNA will be applied in the clinical setting as an effective choice for all pregnant women who opt for safer prenatal diagnostic testing. Eventually, the availability of a reliable non-invasive test to determine fetal T21 would reduce unintended fetal losses and would presumably be welcomed by pregnant women.
Acknowledgments
This article was presented at 17th Seoul International Symposium, Korean Society of Obstetrics and Gynecology, October 4, 2012, and supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A111550).
References
1. Grimshaw GM, Szczepura A, Hulten M, MacDonald F, Nevin NC, Sutton F, et al. Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities. Health Technol Assess 2003;7:1-146. PMID: 12773259.

2. Megarbane A, Ravel A, Mircher C, Sturtz F, Grattau Y, Rethore MO, et al. The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. Genet Med 2009;11:611-616. PMID: 19636252.


3. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin No. 77: screening for fetal chromosomal abnormalities. Obstet Gynecol 2007;109:217-227. PMID: 17197615.


4. Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, et al. First-trimester or second-trimester screening, or both, for Down's syndrome. N Engl J Med 2005;353:2001-2011. PMID: 16282175.


5. Nicolaides KH. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am J Obstet Gynecol 2004;191:45-67. PMID: 15295343.


6. Rozenberg P, Bussieres L, Chevret S, Bernard JP, Malagrida L, Cuckle H, et al. Screening for Down syndrome using first-trimester combined screening followed by second-trimester ultrasound examination in an unselected population. Am J Obstet Gynecol 2006;195:1379-1387. PMID: 16723105.


7. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 88, December 2007. Invasive prenatal testing for aneuploidy. Obstet Gynecol 2007;110:1459-1467. PMID: 18055749.


8. Alfirevic Z, Sundberg K, Brigham S. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database Syst Rev 2003;(3):CD003252PMID: 12917956.


9. Cheung MC, Goldberg JD, Kan YW. Prenatal diagnosis of sickle cell anaemia and thalassaemia by analysis of fetal cells in maternal blood. Nat Genet 1996;14:264-268. PMID: 8896554.


10. Rust DW, Bianchi DW. Microchimerism in endocrine pathology. Endocr Pathol 2009;20:11-16. PMID: 19214801.



11. Bianchi DW, Simpson JL, Jackson LG, Elias S, Holzgreve W, Evans MI, et al. Fetal gender and aneuploidy detection using fetal cells in maternal blood: analysis of NIFTY I data. National Institute of Child Health and Development Fetal Cell Isolation Study. Prenat Diagn 2002;22:609-615. PMID: 12124698.


12. Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet 1997;350:485-487. PMID: 9274585.


13. Lun FM, Chiu RW, Allen Chan KC, Yeung Leung T, Kin Lau T, Dennis Lo YM. Microfluidics digital PCR reveals a higher than expected fraction of fetal DNA in maternal plasma. Clin Chem 2008;54:1664-1672. PMID: 18703764.


14. Lo YM, Zhang J, Leung TN, Lau TK, Chang AM, Hjelm NM. Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet 1999;64:218-224. PMID: 9915961.



15. Lo YM, Chan KC, Sun H, Chen EZ, Jiang P, Lun FM, et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci Transl Med 2010;2:61ra91.


16. Scheffer PG, van der Schoot CE, Page-Christiaens GC, de Haas M. Noninvasive fetal blood group genotyping of rhesus D, c, E and of K in alloimmunised pregnant women: evaluation of a 7-year clinical experience. BJOG 2011;118:1340-1348. PMID: 21668766.


17. Yang YH, Han SH, Lee KR. Noninvasive prenatal diagnosis using cell-free fetal DNA in maternal plasma: clinical applications. J Genet Med 2011;8:1-16.

18. Lim JH, Park SY, Kim SY, Kim DJ, Kim MJ, Yang JH, et al. Effective method for extraction of cell-free DNA from maternal plasma for non-invasive first-trimester fetal gender determination: a preliminary study. J Genet Med 2010;7:53-58.

19. Go AT, van Vugt JM, Oudejans CB. Non-invasive aneuploidy detection using free fetal DNA and RNA in maternal plasma: recent progress and future possibilities. Hum Reprod Update 2011;17:372-382. PMID: 21076134.


20. Chiu RW, Cantor CR, Lo YM. Non-invasive prenatal diagnosis by single molecule counting technologies. Trends Genet 2009;25:324-331. PMID: 19540612.


21. Lo YM, Lun FM, Chan KC, Tsui NB, Chong KC, Lau TK, et al. Digital PCR for the molecular detection of fetal chromosomal aneuploidy. Proc Natl Acad Sci U S A 2007;104:13116-13121. PMID: 17664418.



22. Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc Natl Acad Sci U S A 2008;105:16266-16271. PMID: 18838674.



23. Chiu RW, Chan KC, Gao Y, Lau VY, Zheng W, Leung TY, et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci U S A 2008;105:20458-20463. PMID: 19073917.



24. Sehnert AJ, Rhees B, Comstock D, de Feo E, Heilek G, Burke J, et al. Optimal detection of fetal chromosomal abnormalities by massively parallel DNA sequencing of cell-free fetal DNA from maternal blood. Clin Chem 2011;57:1042-1049. PMID: 21519036.


25. Chiu RW, Akolekar R, Zheng YW, Leung TY, Sun H, Chan KC, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ 2011;342:c7401PMID: 21224326.



26. Ehrich M, Deciu C, Zwiefelhofer T, Tynan JA, Cagasan L, Tim R, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol 2011;204:205.e1205.e11PMID: 21310373.


27. Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP, et al. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol 2012;119:890-901. PMID: 22362253.


28. Palomaki GE, Deciu C, Kloza EM, Lambert-Messerlian GM, Haddow JE, Neveux LM, et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: an international collaborative study. Genet Med 2012;14:296-305. PMID: 22281937.



29. Sparks AB, Struble CA, Wang ET, Song K, Oliphant A. Noninvasive prenatal detection and selective analysis of cell-free DNA obtained from maternal blood: evaluation for trisomy 21 and trisomy 18. Am J Obstet Gynecol 2012;206:319.e1-319.e9. PMID: 22464072.


30. Sparks AB, Wang ET, Struble CA, Barrett W, Stokowski R, McBride C, et al. Selective analysis of cell-free DNA in maternal blood for evaluation of fetal trisomy. Prenat Diagn 2012;32:3-9. PMID: 22223233.



31. Rabinowitz M, Gemelos G, Banjevic M, Ryan A, Demko Z, Hill M. Methods for non-invasive prenatal ploidy calling [Internet]. SumoBrain; c2013. cited 2013 Feb 14. Available from: http://www.sumobrain.com/patents/wipo/Methods-non-invasive-prenatal-ploidy/WO2012108920.html.
32. Natera [Internet]. San Carlos, CA: Natera Inc.; c2013. cited 2013 Feb 14. Available from: http://natera.com.
33. Saito H, Sekizawa A, Morimoto T, Suzuki M, Yanaihara T. Prenatal DNA diagnosis of a single-gene disorder from maternal plasma. Lancet 2000;356:1170PMID: 11030304.


34. Tang NL, Leung TN, Zhang J, Lau TK, Lo YM. Detection of fetal-derived paternally inherited X-chromosome polymorphisms in maternal plasma. Clin Chem 1999;45:2033-2035. PMID: 10545084.


35. Finning K, Martin P, Summers J, Massey E, Poole G, Daniels G. Effect of high throughput RHD typing of fetal DNA in maternal plasma on use of anti-RhD immunoglobulin in RhD negative pregnant women: prospective feasibility study. BMJ 2008;336:816-818. PMID: 18390496.



36. Lo YM, Wong IH, Zhang J, Tein MS, Ng MH, Hjelm NM. Quantitative analysis of aberrant p16 methylation using real-time quantitative methylation-specific polymerase chain reaction. Cancer Res 1999;59:3899-3903. PMID: 10463578.

37. Wong IH, Lo YM, Zhang J, Liew CT, Ng MH, Wong N, et al. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res 1999;59:71-73. PMID: 9892188.

38. Esteller M, Sanchez-Cespedes M, Rosell R, Sidransky D, Baylin SB, Herman JG. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Cancer Res 1999;59:67-70. PMID: 9892187.

39. Poon LL, Leung TN, Lau TK, Chow KC, Lo YM. Differential DNA methylation between fetus and mother as a strategy for detecting fetal DNA in maternal plasma. Clin Chem 2002;48:35-41. PMID: 11751536.


40. Chim SS, Tong YK, Chiu RW, Lau TK, Leung TN, Chan LY, et al. Detection of the placental epigenetic signature of the maspin gene in maternal plasma. Proc Natl Acad Sci U S A 2005;102:14753-14758. PMID: 16203989.



41. Chan KC, Ding C, Gerovassili A, Yeung SW, Chiu RW, Leung TN, et al. Hypermethylated RASSF1A in maternal plasma: A universal fetal DNA marker that improves the reliability of noninvasive prenatal diagnosis. Clin Chem 2006;52:2211-2218. PMID: 17068167.


42. Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature 1993;366:362-365. PMID: 8247133.


43. Driscoll DJ, Waters MF, Williams CA, Zori RT, Glenn CC, Avidano KM, et al. A DNA methylation imprint, determined by the sex of the parent, distinguishes the Angelman and Prader-Willi syndromes. Genomics 1992;13:917-924. PMID: 1505981.


44. Schneid H, Seurin D, Vazquez MP, Gourmelen M, Cabrol S, Le Bouc Y. Parental allele specific methylation of the human insulin-like growth factor II gene and Beckwith-Wiedemann syndrome. J Med Genet 1993;30:353-362. PMID: 8320696.



45. Gama-Sosa MA, Midgett RM, Slagel VA, Githens S, Kuo KC, Gehrke CW, et al. Tissue-specific differences in DNA methylation in various mammals. Biochim Biophys Acta 1983;740:212-219. PMID: 6860672.


46. Fuke C, Shimabukuro M, Petronis A, Sugimoto J, Oda T, Miura K, et al. Age related changes in 5-methylcytosine content in human peripheral leukocytes and placentas: an HPLC-based study. Ann Hum Genet 2004;68:196-204. PMID: 15180700.


47. Maccani MA, Marsit CJ. Epigenetics in the placenta. Am J Reprod Immunol 2009;62:78-89. PMID: 19614624.



48. Bianchi DW. Fetal DNA in maternal plasma: the plot thickens and the placental barrier thins. Am J Hum Genet 1998;62:763-764. PMID: 9529367.



49. Flori E, Doray B, Gautier E, Kohler M, Ernault P, Flori J, et al. Circulating cell-free fetal DNA in maternal serum appears to originate from cyto- and syncytio-trophoblastic cells. Case report. Hum Reprod 2004;19:723-724. PMID: 14998976.


50. Lui YY, Chik KW, Chiu RW, Ho CY, Lam CW, Lo YM. Predominant hematopoietic origin of cell-free DNA in plasma and serum after sex-mismatched bone marrow transplantation. Clin Chem 2002;48:421-427. PMID: 11861434.


51. Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, et al. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A 1992;89:1827-1831. PMID: 1542678.



52. Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A 1996;93:9821-9826. PMID: 8790415.



53. Grunau C, Clark SJ, Rosenthal A. Bisulfite genomic sequencing: systematic investigation of critical experimental parameters. Nucleic Acids Res 2001;29:E65-E65. PMID: 11433041.



54. Tong YK, Jin S, Chiu RW, Ding C, Chan KC, Leung TY, et al. Noninvasive prenatal detection of trisomy 21 by an epigenetic-genetic chromosome-dosage approach. Clin Chem 2010;56:90-98. PMID: 19850629.


55. McClelland M, Nelson M, Raschke E. Effect of site-specific modification on restriction endonucleases and DNA modification methyltransferases. Nucleic Acids Res 1994;22:3640-3659. PMID: 7937074.



56. Weisenberger DJ, Trinh BN, Campan M, Sharma S, Long TI, Ananthnarayan S, et al. DNA methylation analysis by digital bisulfite genomic sequencing and digital Methy-Light. Nucleic Acids Res 2008;36:4689-4698. PMID: 18628296.



57. Li M, Chen WD, Papadopoulos N, Goodman SN, Bjerregaard NC, Laurberg S, et al. Sensitive digital quantification of DNA methylation in clinical samples. Nat Biotechnol 2009;27:858-863. PMID: 19684580.



58. Lim JH, Kim SY, Park SY, Lee SY, Kim MJ, Han YJ, et al. Non-invasive epigenetic detection of fetal trisomy 21 in first trimester maternal plasma. PLoS One 2011;6:e27709PMID: 22132128.



59. Chim SS, Jin S, Lee TY, Lun FM, Lee WS, Chan LY, et al. Systematic search for placental DNA-methylation markers on chromosome 21: toward a maternal plasma-based epigenetic test for fetal trisomy 21. Clin Chem 2008;54:500-511. PMID: 18202156.


60. Tong YK, Chiu RW, Akolekar R, Leung TY, Lau TK, Nicolaides KH, et al. Epigenetic-genetic chromosome dosage approach for fetal trisomy 21 detection using an autosomal genetic reference marker. PLoS One 2010;5:e15244PMID: 21249119.



61. Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, et al. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet 2005;37:853-862. PMID: 16007088.


62. Khulan B, Thompson RF, Ye K, Fazzari MJ, Suzuki M, Stasiek E, et al. Comparative isoschizomer profiling of cytosine methylation: the HELP assay. Genome Res 2006;16:1046-1055. PMID: 16809668.



63. Down TA, Rakyan VK, Turner DJ, Flicek P, Li H, Kulesha E, et al. A Bayesian deconvolution strategy for immunoprecipitation-based DNA methylome analysis. Nat Biotechnol 2008;26:779-785. PMID: 18612301.



64. Weng YI, Huang TH, Yan PS. Methylated DNA immunoprecipitation and microarray-based analysis: detection of DNA methylation in breast cancer cell lines. Methods Mol Biol 2009;590:165-176. PMID: 19763503.



65. Papageorgiou EA, Karagrigoriou A, Tsaliki E, Velissariou V, Carter NP, Patsalis PC. Fetal-specific DNA methylation ratio permits noninvasive prenatal diagnosis of trisomy 21. Nat Med 2011;17:510-513. PMID: 21378977.



-
METRICS

-
- 9 Crossref
- 3,243 View
- 34 Download
- Related articles in Obstet Gynecol Sci
-
Prenatal diagnosis by isolation of fetal nucleated RBCs in maternal peripheral blood.2007 June;50(6)