Introduction
With recent advances in fetal medicine, the concept of the fetus as a patient has led to the application of treatments in utero. The aim of fetal therapy is to save fetuses who are facing perinatal death or devastating consequences despite optimal management after birth. The first fetal intrauterine blood transfusion, performed in the early 1960s, is thought to mark the beginning of fetal therapy [1]. The milestones of fetal surgical therapy are shown in Table 1. With advances in ultrasound imaging in the 1980s, fetal blood transfusion [2], vesicoamniotic shunting for lower urinary tract obstruction (LUTO) [3], and thoracoamniotic shunting (TAS) for hydrothorax [4] were performed with ultrasound guidance. Pediatric surgeons at the University of California at San Francisco (UCSF) introduced open fetal surgery for performing direct surgical procedures on fetuses with hysterotomy for LUTO [5], congenital pulmonary airway malformations (CPAM) [6], and congenital diaphragmatic hernia (CDH) [7]. In the 1990s, fetoscopic laser surgery (FLS) for twin-twin transfusion syndrome (TTTS) was first performed, showing great success [8-11]. Open fetal surgery for CDH was changed to a fetoscopic procedure and established as fetoscopic endoluminal tracheal occlusion (FETO) in the 2000s [12,13]. The revival of open fetal surgery began in the 2010s after a successful clinical study for myelomeningocele (MMC) [14].
Table┬Ā1
Milestones of surgical fetal therapy
CPAM, congenital pulmonary airway malformation; CDH, congenital diaphragmatic hernia; EXIT, ex utero intrapartum treatment; FETENDO, fetal endoscopic surgery; FETO, fetoscopic endoluminal tracheal occlusion; FLS, fetoscopic laser surgery; MMC, myelomeningocele; MOMS, management of myelomeningocele study; RFA, radiofrequency ablation; TRAP, twin reversed arterial perfusion.
After 50 years, a number of fetal therapies are now being performed as clinically useful treatments, although others are still considered experimental treatments. Most achievements in fetal therapy have been made in the US and Western Europe [15]. We in Japan have contributed a bit through the promotion of fetal therapy in prenatal care in our country. Indeed, our findings have led three fetal therapiesŌĆöFLS for TTTS [16], TAS for hydrothorax, and radiofrequency ablation (RFA) for twin reversed arterial perfusion (TRAP) sequenceŌĆöto be approved for coverage by Japan National Health Insurance (Table 2). This means that these three fetal therapies are recognized as standard prenatal care, and fetuses are recognized as patients by the administration.
Table┬Ā2
Fetal therapies covered by Japan National Health Insurance
| Disorder | Treatment | Approval | References of our study |
|---|---|---|---|
| Twin-twin transfusion syndrome | Fetoscopic laser surgery | Apr-12 | Sago et al. [22] |
| Primary fetal hydrothorax | Thoraco-amniotic shunting | Jul-12 | Takahashi et al. [43] |
| Twin reversed arterial perfusion sequence | Radiofrequency ablation | Mar-19 | Sugibayashi et al. [56] |
| Wagata et al. [57] |
We herein review the present state of fetal therapy to highlight its role in perinatal medicine as standard prenatal care.
Classification of fetal therapy
Fetal therapy consists of medical therapy and surgical therapy, including percutaneous ultrasound-guided surgery, fetoscopic surgery, and open fetal surgery [17]. Medical fetal therapy involves the administration of medicine to the mother, where it is then transported to the fetus via the placenta or amniotic fluid. Fetal pharmacotherapy is used to manage arrhythmias, especially tachyarrhythmias, congenital adrenal hyperplasia and thyroid disorders [17]. Percutaneous ultrasound-guided surgery involves the insertion of a needle or catheter via the mother's abdomen into the fetus under ultrasound guidance. Percutaneous surgery includes shunt therapy for LUTO or hydrothorax, RFA for TRAP sequence, and intracardiac catheter procedures for aortic or pulmonary valvuloplasty for stenosis. Fetoscopic surgery involves placing an endoscope in the amniotic fluid space for treatments. FLS uses a laser to coagulate the placental vessels, and FETO places a detachable balloon in the trachea of the fetus. Open fetal surgery is surgery performed directly on the fetus by laparotomy and hysterotomy, after which the hysterotomy is closed to keep the fetus in the uterus. Of note, it is associated with extensive morbidities for both the mother and the fetus. Most cases of open fetal surgery are performed for MMC.
Assessment of fetal surgical therapy
To assess the effectiveness of therapy, good-quality evidence is required. However, clear evidence in the field of fetal therapy is limited because of the rarity of the targeted diseases and difficulties enrolling unborn patients. Only three randomized control trials (RCTs) have suggested any benefits of fetal treatments thus far [11,14,18]. Two of these RCTs were for FLS, while the other was for open fetal surgery for MMC. We assessed the effectiveness of select fetal surgical therapies by scoring them AA to C (AA: effective according to RCT, A: effective, B: expected, C: unknown) based on previous reports and our own experience (Table 3). The availability of fetal therapy in Japan is also presented.
Table┬Ā3
Assessments of select fetal surgical therapies and their availability in Japan
AA, effective by randomized control trial; A, effective; B, expected; C, unknown; N, no; Y, yes.
*Only one case underwent [58].
Percutaneous intracardiac catheter procedures for critical aortic stenosis, fetoscopic laser for LUTO, or open fetal surgery for MMC have not yet been performed in Japan, although we are preparing to perform them in the near future. The costs of three fetal therapies (FLS for TTTS, TAS for hydrothorax, and RFA for TRAP sequence) are compensated by Japan National Health Insurance. FLS for TTTS is the most common fetal therapy performed, followed by TAS for hydrothorax and RFA for TRAP sequence. These three minimally invasive fetal therapies constitute 85% of all treatments for fetuses performed at our center [16].
Fetoscopic laser surgery
TTTS, which occurs in one-tenth of monochorionic (MC) twin pregnancies, has high perinatal morbidity and mortality [19]. TTTS is diagnosed in cases of MC twin pregnancy complicated by polyhydramnios with a maximum vertical pocket (MVP) Ōēź8.0 cm (with an enlarged bladder) in the recipient and oligohydramnios with an MVP Ōēż2.0 cm (with a small bladder) in the donor [20]. TTTS is considered to be a hemodynamic and likely hormonal disorder derived from a chronic blood flow imbalance between twins by vascular anastomoses on the placenta [21].
FLS, which ablates placental vascular anastomoses, is an effective treatment option for TTTS. The original technique of FLP was first reported by De Lia et al. with laparotomy [8] and advanced by Ville et al. as a percutaneous procedure under ultrasound guidance [9,10]. The RCT by the Eurofoetus group showed that FLS is more useful than serial amnioreduction [11]. FLS is widely accepted and performed as a standard treatment for TTTS around the world [16].
We started the FLS program in Japan in 2002. We reported our initial 181 cases of TTTS treated by FLS at 4 centers [22]. The mean gestational ages at FLS and delivery were around 21 and 33 weeks, respectively. The survival rates of both twins and at least 1 twin at 6 months of age were 62% and 90%, respectively. Our findings led to recognition of FLS as the first-line treatment option for TTTS, and the costs of this procedure have been compensated by Japan National Health Insurance since April 2012 [16]. FLS was the first fetal therapy to be compensated by Japan National Health Insurance and is now established as a standard prenatal care procedure.
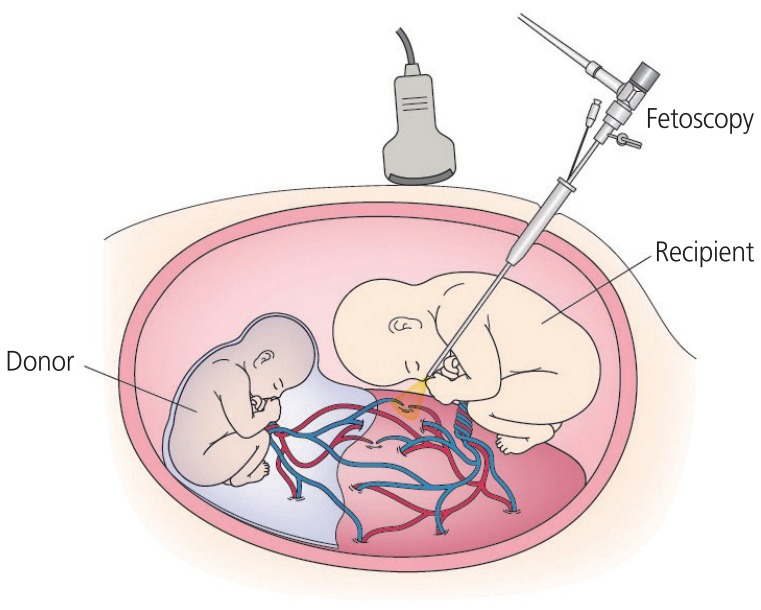
The procedure is illustrated in Fig. 1, and the details are described in our review [16]. A recent modified version of FLS, referred to as the ŌĆśSolomon technique,ŌĆÖ adds ablation of the placenta to connect the anastomosesŌĆÖ coagulation sites in order to diminish residual anastomoses [18]. The perinatal outcomes after FLS improved significantly with advances in the technique and learning [23]. The recent survivals of both twins and at least one twin have been reported to be 70% and >90%, respectively [16]. However, despite this ameliorated survival rate, TTTS treated by FLS is still associated with neurodevelopmental impairments, including severe cerebral palsy and neurodevelopment delay. There is still about a 10% risk of long-term neurodevelopment impairment after FLS [16]. Improvement in the neurodevelopmental outcomes of twins treated with FLP is a future point of focus.
Fig.┬Ā1
A schematic representation of fetoscopic laser surgery for twin-twin transfusion syndrome. A fetoscope is percutaneously inserted into the recipient sac through a cannula. Placental vascular anastomoses between twins are ablated by a laser.
FLS is offered as the standard treatment option for TTTS at 16-26 weeksŌĆÖ gestation, which is considered to be the conventional indication [11,16]. Fetofetal transfusion syndrome (FFTS), which induces perinatal death and neurodevelopmental impairment, occurs in dichorionic triamniotic (DT) or monochorionic triamniotic (MT) triplets [24]. FFTS in triplets accords with TTTS in twins, so FLS is supposed to be a viable treatment option for FFTS in triplets. We and others have shown that FLS is a useful therapy for FFTS in both DT and MT triplets [25,26]. The optimum gestational age for the therapy of TTTS was set at 16-26 weeks in the Eurofoetus multicenter randomized trial for FLS [11]; however, cases of TTTS after 26 weeks tend to demonstrate a high rate of morbidities despite receiving optimal management after birth. The accumulated results suggest that FLS could be a safe treatment option for TTTS after 26 weeks, especially at 26-28 weeks of gestation [27-29].
With the enhanced awareness of TTTS, more attention is now being paid to selective intrauterine growth restriction (sIUGR), a complicated MC twin pregnancy. sIUGR is diagnosed according to the difference in the fetal body weight of twins. Several studies have shown the application of FLS for sIUGR with absent or reverse end-diastolic velocity (AREDV) in the umbilical artery (UA) [30-32]. FLS may be a promising treatment option for sIUGR when accompanied by both AREDV in UA and severe oligohydramnios of IUGR twins [33,34]. Oligohydramnios in the IUGR twin may be an essential indication for FLP for sIUGR. The FLS criteria for non-conventional applications, including the treatment of triplets, TTTS after 26 weeks, and sIUGR, are shown in Table 4 [16]. FLS is the most successful and commonly performed fetal therapy at present and is recognized as a standard prenatal care approach, despite FLS only being available at select fetal treatment centers.
Table┬Ā4
Our criteria for performing fetoscopic laser surgery
Thoracoamniotic shunting
Fetal hydrothorax (FHT) is a form of pleural effusion seen in fetuses. Primary FHT, called isolated FHT, is chylothorax due to lymphatic leakage. Secondary FHT is pleural fluid accumulation associated with fetal diseases, including fetal anemia, viral infection, and structural and chromosomal abnormalities [35-37]. The prognosis of secondary FHT relies on the primary etiology. The incidence of primary FHT is approximately 1/15,000 [38]. Its natural history varies from spontaneous disappearance to the progressive aggregation of fluid and the occurrence of hydrops. Fetuses with primary FHT carry poor prognoses when they develop hydrops, which is thought to be a consequence of decreasing venous return to the heart affected by massive pleural effusion. TAS, which drains the pleural effusion, is a treatment option to improve the perinatal prognoses and has been reported to be associated with a better outcome in severe primary FHT than no fetal intervention [39-41].
A ŌĆ£double-basket catheterŌĆØ (Hakko Co., Nagano, Japan) was invented in Japan in the 1990s [42] and has since been frequently used for TAS in Japan. The procedure is illustrated in Fig. 2. We performed a prospective one-arm trial of TAS for isolated FHT using this very thin double-basket catheter [43]. The median gestational age at the procedure and delivery was about 27 and 35 weeks, respectively. The overall survival rate was 79%, and the survival rate in the hydropic fetuses was 71%. Drainage of fetal pleural effusions with a double-basket catheter was shown to be safe and effective. Our results led to the approval of the ŌĆ£double-basket catheterŌĆØ as a medical device, and the costs of TAS have been compensated by Japan National Health Insurance since July 2012. TAS was the second fetal therapy to be compensated by Japan National Health Insurance and is now established as a standard prenatal care approach.
Fig.┬Ā2
A schematic representation of thoracoamniotic shunting for fetal hydrothorax. A double-basket catheter is placed in the chest to drain the pleural effusion into the amniotic fluid.

We conducted a nationwide survey on fetuses with primary FHT delivered after 22 weeks' gestation at January 2007 to December 2011 [44]. The survivals for primary FHT with and without hydrops were 58% and 98%, respectively. A lower gestational age at the diagnosis, severe hydrops and severe pleural effusion were predicting factors of poor prognoses. TAS was associated with a significant reduction in the risk of death in hydropic cases. Down syndrome, or trisomy 21, is frequently associated with FHT. The prognosis of FHT with trisomy 21 is not very bad but is still poorer than that of primary FHT [45]. Fetal therapy is thought to provide no benefits for FHT with trisomy 21 [45].
Radiofrequency ablation
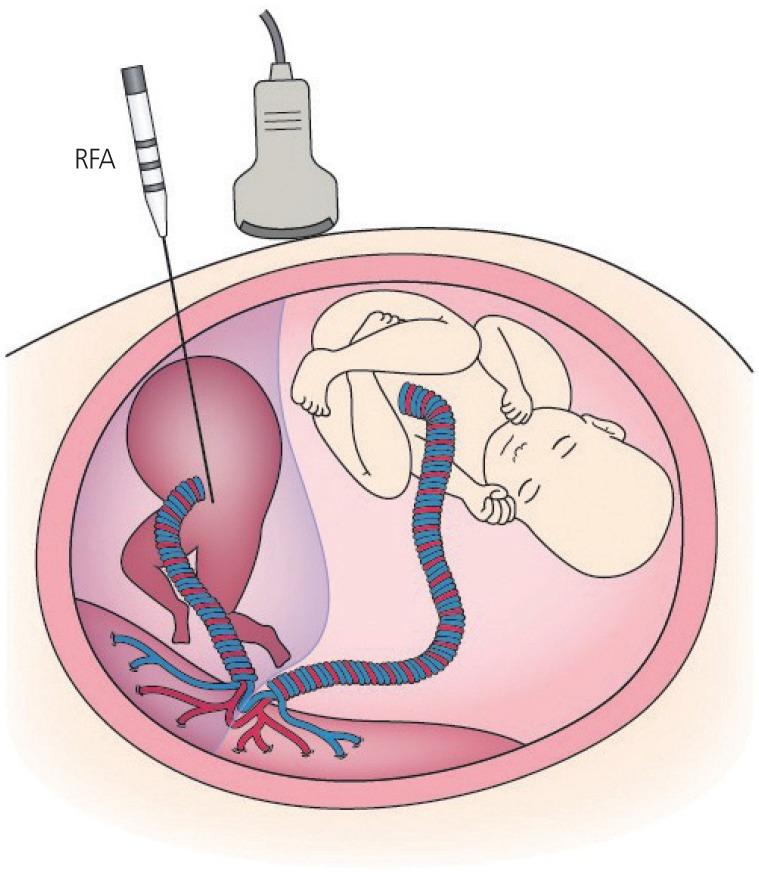
An acardiac twin is an uncommon complicated monochorionic twin pregnancy occurring in 1% of MC twins [46]. The acardiac twinŌĆÖs blood is circulated by the pump twin through arterioarterial and venovenous anastomoses on the placenta. The perinatal outcome is particularly poor in cases with continuing reversed arterial flow, called TRAP sequence, as the pump twin develops high-output cardiac failure, leading to fetal hydrops and/or intrauterine fetal death (IUFD) or preterm birth due to polyhydramnios [47]. Various fetal therapies for interrupting the perfusion of the acardiac twin have been undertaken. These procedures include extrafetal treatment, such as monopolar or bipolar cord coagulation [48,49], and intrafetal treatment, such as laser ablation [50] and RFA [51], which are minimally invasive techniques.
RFA induces coagulation of tissue by delivering high temperatures through a high-frequency alternating current. The first case of TRAP sequence treatment using RFA was shown by the University of California at San Francisco [52]. RFA uses a 17-gauge needle electrode guided by ultrasound. The procedure is illustrated in Fig. 3. RFA has a lower risk of premature preterm rupture of membranes than extrafetal methods [53,54]. Lee et al. [55] reported the North American Fetal Therapy Network registry data of RFA treatment for TRAP sequence and showed the 80% survival rate. Two studies of RFA for TRAP sequence were performed in Japan [56,57]. We reported the outcomes of 40 cases of TRAP sequence treated with RFA, which is the largest single-center study [56]. The overall survival rate was 85%, and the mean gestational age at delivery was 37 weeks for the pump twins. The other study also reported good outcomes of RFA treatment for TRAP sequence, with a survival rate of 88% [57]. Although reports of RFA treatment for TRAP sequence are limited, RFA is thought to be a useful treatment option for TRAP sequence after 15 weeks' gestation. Our results led to the approval of a medical device application for RFA, and the costs of RFA are now compensated by Japan National Health Insurance as of March 2019. RFA for TRAP sequence is the third fetal therapy to be compensated by Japan National Health Insurance and is considered a standard prenatal care approach for TRAP sequence.
Conclusion
More than 50 years have passed since the first attempt to treat a fetus in utero. However, while various efforts have been made, many fetal therapies are still considered experimental. Nevertheless, some fetal therapies are now accepted as effective treatments options, including FLS for TTTS, TAS for primary FHT, and RFA for TRAP sequence. These 3 fetal therapies have been approved for coverage by Japan National Health Insurance based on the results of clinical studies performed in Japan. FLS for TTTS, TAS for primary FHT, and RFA for TRAP sequence are all minimally invasive fetal therapies that have become standard prenatal care approaches in Japan. These 3 fetal therapies will help improve the perinatal outcomes of fetuses with these diseases. The goal of fetal therapy is to ensure the survival of fetuses without complications. Well-designed studies and cautious preparations are essential for effectively promoting fetal therapy.