Introduction
A brief history of external cephalic version
Timing of external cephalic version
Contraindications to external cephalic version
ŌĆó Onset of active labor: Uterine contractions caused by active labor make it difficult to perform an ECV trial. However, ECV can be attempted during the time interval between uterine contractions. Two studies that described intrapartum ECV reported promising results with success rates of 73% (11 of 15 attempts) [20] and 92.3% (12 of 13 attempts) [21]. Therefore, onset of active labor is not an absolute contraindication for ECV.
ŌĆó Rupture of membranes: Most clinicians regard rupture of membranes as a relative contraindication for ECV because decreased amniotic fluid volume itself hinders the trial [15]. However, ECV trials may be successfully performed in multiparous women with some amount of amniotic fluid. Thus, rupture of membranes is not an absolute contraindication for ECV.
ŌĆó Vaginal bleeding: It is preferable to delay ECV or completely avoid the procedure in a woman with recent vaginal bleeding of unknown origin or bleeding secondary to placental detachment [15].
ŌĆó Severe preeclampsia or eclampsia: Fetuses of women with this condition commonly develop heart rate deceleration after ECV. A previous study reported that nearly 50% of the fetuses evaluated in that study showed abnormal heart rate tracings during or immediately after the procedure [22], which suggests that although transient, ECV is a stressful event for the fetus [15].
ŌĆó Multiple gestation: Twin fetuses are known to easily return to breech presentation after successful ECV. Furthermore, an ECV trial in such cases can cause rupture of the intertwin membrane, which can progress to serious complications such as amniotic band or cord entanglement. Therefore, most clinicians do not attempt ECV in women with multiple pregnancies.
ŌĆó Major fetal anomalies: It is preferable to avoid ECV in women in whom prenatal ultrasonography reveals major fetal anomalies such as complex cardiac defects, significant brain malformation, and anomalies affecting the fetal pulmonary system. However, an ECV trial is not contraindicated in women in whom minor fetal anomalies are detected.
ŌĆó Abnormal cardiotocography before external cephalic version: Most authors agree that ECV should not be performed in cases of abnormal EFM before the trial because the procedure may aggravate the situation.
ŌĆó Growth restriction associated with an abnormal umbilical artery Doppler index: Fetuses with this condition commonly show abnormal heart rates after the ECV trial [15]. Therefore, it is safe to avoid the procedure in these cases.
ŌĆó Previous cesarean section: An ECV trial can be attempted in women who wish to trial vaginal birth after previous cesarean section. This practice is based on the recommendations of a study in which ECV was performed on 42 women with term breech presentation with a history of previous cesarean section, with a success rate of 74.0% without significant fetal or maternal complications [23]. Similarly, another prospective, comparative cohort study reported 74 ECV trials performed on women with a history of cesarean section with a 67.1% success rate, without significant complications [24].
ŌĆó Nuchal cord: No guidelines or definitive data are available regarding the risk of ECV in women with ultrasonographic evidence of nuchal cord. Based on the author's personal experience of performing >1,000 consecutive ECV trials (unpublished data), a single nuchal cord does not increase the risk; however, >2 tight nuchal cords tend to decrease the success rate of ECV.
Factors associated with successful external cephalic version
ŌĆó Parity: Multiparous women tend to show a higher ECV success rate (72.3%) than nulliparous women (between 40% and 64%) [26,28,30,31].
ŌĆó Amniotic fluid: It has been reported that an amniotic fluid index (AFI) >7 cm is associated with a successful ECV trial [26,30,31,32]; however, a study reported significantly higher success rates with AFI >10 (odds ratio [OR], 1.8; 95% confidence interval [CI], 1.5-2.1) [33]. Furthermore, a systematic review described a low ECV success rate with low amniotic fluid volume, although it was not possible to define the AFI threshold associated with an unsuccessful ECV trial [34].
ŌĆó Non-anterior placental location: ECV trials were more successful in women with the placenta located on the posterior uterine wall (OR, 1.9; 95% CI, 1.5-2.4) [33], and an anteriorly located placenta decreased the success rate of the ECV [33,34]. This was probably because an anterior placenta hinders palpation of the fetal head, and most clinicians do not exert full pressure when turning the fetus to avoid the risk of abruption of an anteriorly placed placenta.
ŌĆó Type of breech presentation: Frank breech presentation is associated with a lower rate of success [30] than a complete breech presentation (OR, 2.3; 95% CI, 1.9-2.8) [33], possibly because in cases of frank breech presentation, the fetal buttocks are often engaged deeply and are firmly secured within the maternal pelvis; thus, lifting the buttocks is more difficult compared to other types of breech presentations.
ŌĆó Estimated fetal weight before external cephalic version: The effect of estimated fetal weight on the success rates of ECV is controversial in that a few authors have reported greater success rates with high estimated fetal weight [26,31], whereas a few others have reported no such association [35].
ŌĆó Maternal body mass index (BMI): A cross-sectional analysis of 51,002 ECV trials in the United States showed lower success rates with high maternal BMI (P<0.01). The success rate was 58.5% in women with BMI Ōēź40 kg/m2 and 65% in women with normal BMI [36].
Cesarean section after successful external cephalic version
Complications
ŌĆó Transient abnormal cardiotocography patterns: With an incidence rate of 5.7-9.6%, a transient abnormal cardiotocography pattern is the most commonly reported complication of ECV [43]. Temporary baseline bradycardia is relatively common (9-9.6%) [44,45]; however, a report from Japan has described an exceptionally high incidence of fetal bradycardia during or immediately after ECV (48.5%, 189 of 390 cases) that lasted for <1 minute in 43.3%, <5 minutes in 88.9%, and <10 minutes in 98.4% of cases [22]. Bradycardia lasting >10 minutes occurred in 3 of 390 cases (0.7%), and low Apgar scores at 5 minutes, with an umbilical cord arterial pH <7.1 were observed in 2 of these cases [22].
ŌĆó Fetal heart rate deceleration: Although the incidence of a persistent pathological cardiotocographic pattern was between 0.2% (2 of 980 trials) [44] and 0.37% [43] after ECV, it was not significantly associated with the AFI [32].
ŌĆó Umbilical cord accidents: Success or failure of ECV was not associated with an increased risk of umbilical cord accidents [45].
ŌĆó Placental abruption: A recent systematic review of 7,377 ECV trials reported a placental abruption incidence rate of 0.12% [43]; however, an earlier systematic review of 2,503 ECV trials published in 2006 identified no reports of placental abruption associated with ECV [46].
ŌĆó Vaginal bleeding: Vaginal spotting or bleeding occurred in 0.47% of women after the procedure [43]. Although the cause of vaginal bleeding is unclear, it can infrequently be associated with placental abruption. Therefore, in the event of vaginal spotting, the ECV trial should be promptly discontinued, and close monitoring for further spotting and cardiotocography should be performed.
ŌĆó Premature rupture of the membranes: A previous study has reported that immediate delivery occurred in 1.3% of women following ECV secondary to premature rupture of membranes [47]; however, a systematic review of 2,503 ECV trials reported no such findings [46].
ŌĆó Fetomaternal hemorrhage: The rate of detectable fetomaternal hemorrhage during ECV was reported to be 2.4% [48]. Moreover, estimated fetomaternal hemorrhage >30 mL occurred in just 1 case (0.08%) reported among the 1,311 ECV trials; therefore, it was recommended that Rh immunoglobulin in addition to the routine dose of 300 microgram dose need not be administered at 28 weeks of gestation and postpartum [48].
ŌĆó Uterine rupture: Uterine rupture during an ECV trial is one of the most catastrophic complications. However, fortunately, this is rare, and no cases included in a systematic review of 2,503 ECV trials have reported this complication [46].
ŌĆó Emergency cesarean section: The risk of emergency cesarean deliveries during and after ECV reportedly ranges between 0.2% and 0.7% [15,22,43,44,49], with the most common indication being an abnormal fetal heart rate pattern detected on EFM.
ŌĆó Fetal death: A previous study reported no increase in the risk of antepartum fetal death associated with ECV [46], whereas another study reported that although no intrauterine death occurred within 24 hours of performing ECV, intrauterine death occurred in 1 case (0.09%) at 4 weeks following an uncomplicated ECV [50]. Furthermore, a recent cohort study spanning over 18 years reported a corrected post-ECV perinatal mortality rate of 0.12% [28], whereas a systematic review of 7,377 women reported a slightly higher perinatal mortality rate of 0.16% [43]. Another Cochrane systematic review published in 2015 reported that perinatal mortality occurred in 2 of 644 neonates in the ECV group and in 6 of 661 neonates in the control group [1].
Procedure of external cephalic version
1. Before performing the external cephalic version
1) Obtaining pre-procedural informed consent
2) Electronic fetal monitoring
2. Technique of external cephalic version
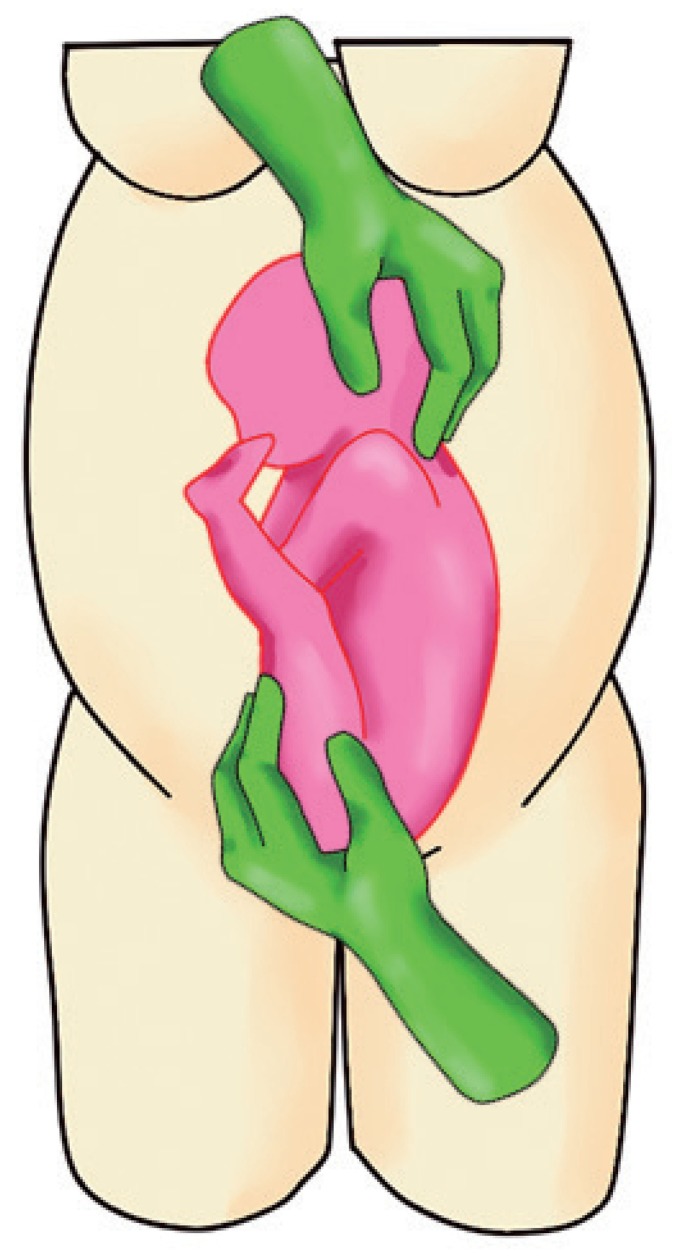
a. Dislodge the fetal buttocks from the pelvis pushing upwards and subsequently laterally with 1 hand (usually the dominant one) (Fig. 1).
b. Gently grasp the fetal head and direct it downwards with the other hand (Fig. 1).
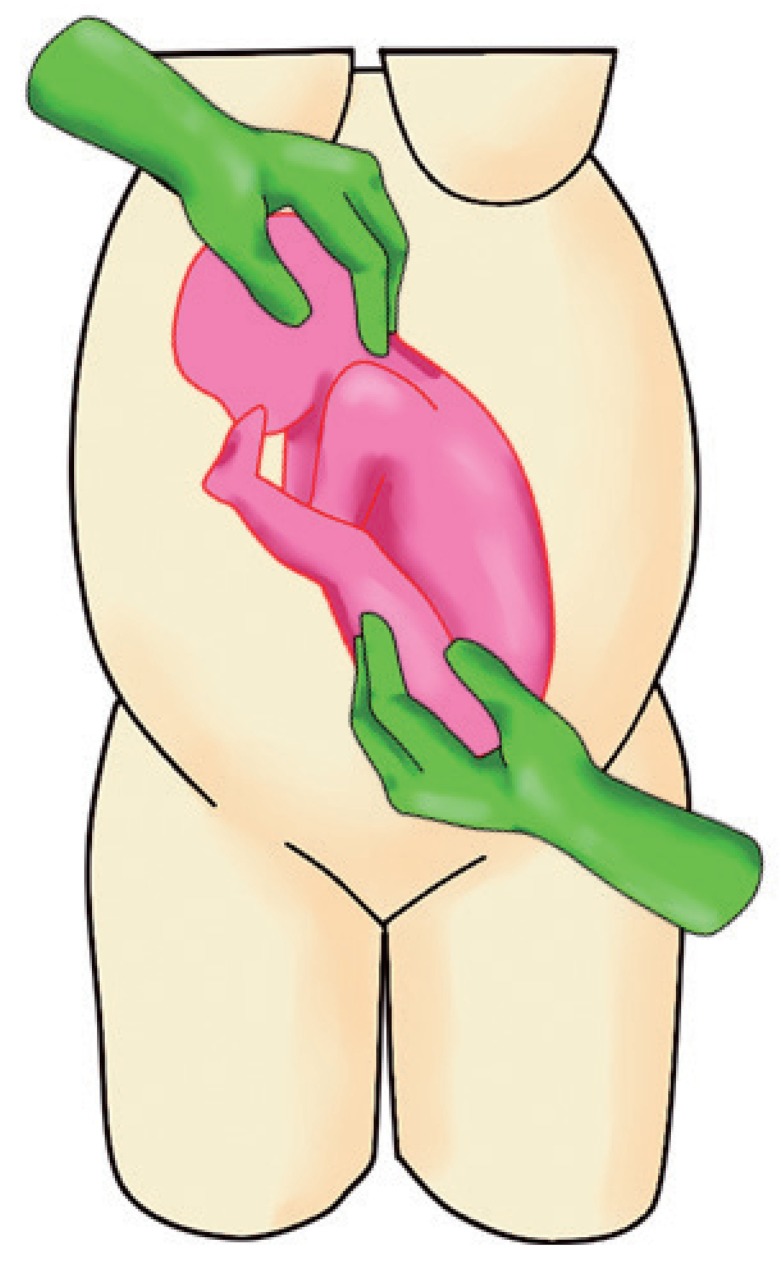
c. Slowly rotate the fetus by pushing upwards (applying 70% of pushing power) and simultaneously guide the head downwards (applying 30% of pushing power) (Fig. 2).
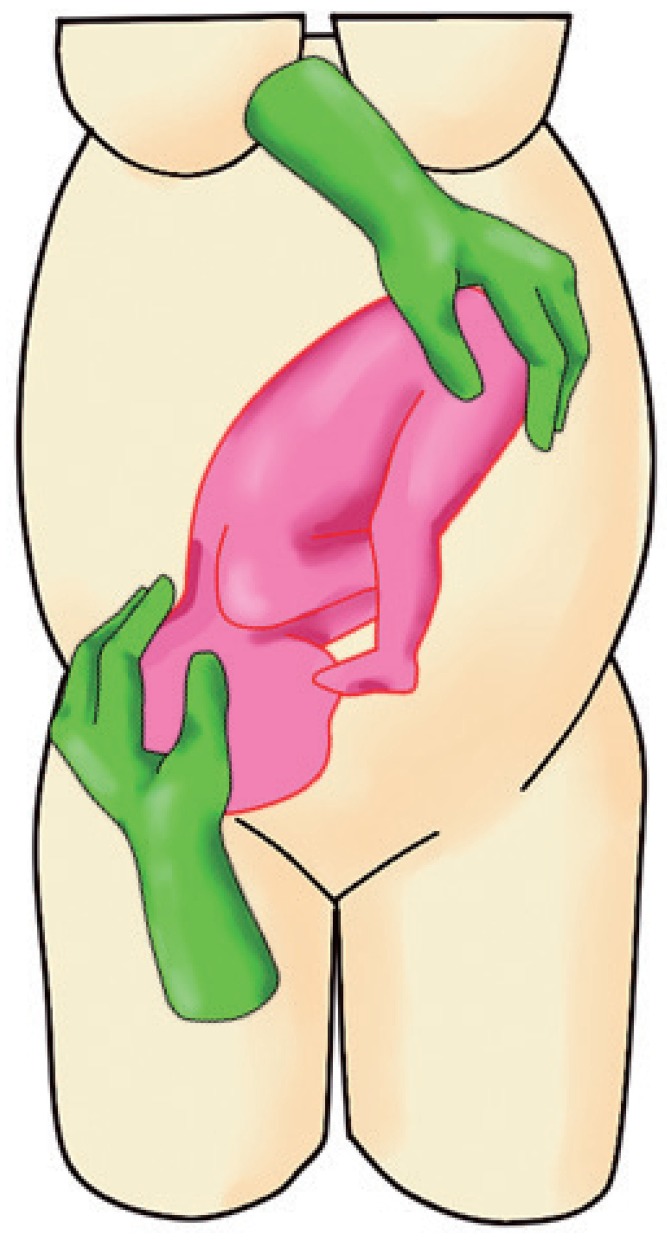
d. When the head is below the level of the fetal buttocks, direct the head securely into the maternal pelvis. This is to ensure that the fetal head does not revert to its original (breech) position (Fig. 3).
e. If the forward roll fails, a backward flip can be tried in the opposite direction of the fetal occiput descending first.
f. The ECV procedure can be performed either by a single operator or with the help of an assistant (to help with pushing the buttocks upward or with performing ultrasonography during the procedure).
g. Discontinue the procedure if the woman complains of significant discomfort or if the fetal heart rate is atypical or abnormal.
h. If the fetal heart rate does not recover within 3 minutes of emergency measures (maternal lateral position, bolus fluid infusion, and/or oxygen mask placement, among other such measures), it is necessary to prepare for an emergency cesarean section. If the fetal heart rate does not recover within 5 minutes, an emergency cesarean section is performed. Delivery should be completed within 10 minutes after the onset of bradycardia [22].