Correlation between the posterior vaginal wall and apex in pelvic organ prolapse
Article information
Abstract
Objective
The aim of our study is to reveal the correlation between the posterior vaginal wall and apex in pelvic organ prolapse.
Methods
We retrospectively reviewed the records of all new patient visits to a urogynecology clinic between January 2013 and December 2015.
Results
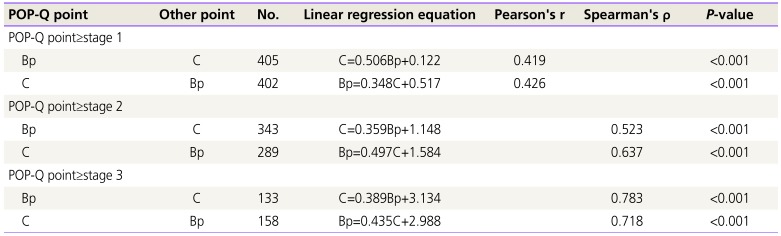
Four hundred five cases were enrolled in our study. When all POP stages were included, the Bp (pelvic organ prolapse quantification point) had a moderate correlation with the C (Pearson's r=0.419; P<0.001). Cases where Bp was stage 3 and above presented strong positive correlations with C (Spearman's ρ=0.783; P<0.001). Cases where C was stage 3 and above presented also strong positive correlations with Bp (Spearman's ρ=0.718; P<0.001).
Conclusion
Posterior vaginal wall prolapse and apical prolapse were correlated with each other, and this correlation was more prominent as stage increased. Therefore, when admitting a patient suspected of posterior vaginal wall prolapse or apical prolapse, it is necessary to evaluate both conditions. Especially in cases more severe or equal to stage 3, it is a must to suspect both conditions as the 2 are strongly correlated.
Introduction
Pelvic organ prolapse (POP) is a pelvic floor disorder, where a segment, segments, or the entire vagina herniates through the vaginal introitus. The nature of POP relates to the structural support of the vagina and uterus, with the apical compartment determined by relatively long vertical fibers that involve the cervix and upper vagina, while midvaginal support of the anterior and posterior compartments is determined by shorter and more direct connections. There are many different combinations of vaginal support defects in POP. These involve the anterior vaginal wall (Ba), posterior vaginal wall (Bp), and apex (C) [1].
POP requires surgery in approximately 200,000 American women each year, making it the pelvic floor disorder most often requiring surgical repair [2]. Reoperation of POP is also common. One recent study reported a POP recurrence rate of 58% one year after the operation, and approximately 17% of the patients treated with POP repair underwent reoperation within 10 years, with the majority of these procedures directed at the same site as the previous repair [34].
The cause of POP recurrence is not well understood, but it is thought to be multifactorial. Underlying connective tissue and neuromuscular differences may predispose to recurrence, and individual procedures may not adequately treat all vaginal compartments, which predisposes to surgical recurrence in another compartment. In addition, all vaginal wall defects may not be recognized before the operation, which leads to a persistent vaginal wall defect. Due to the causes mentioned above, recent studies have focused on the correlation between each vaginal compartment. Among these studies, many have shown positive correlation between the anterior vaginal wall and apex [567]. However, there have not been many studies focusing on apical and posterior vaginal wall support to evaluate the interactions between these compartments in the formation of the clinical problem referred to as uterine prolapse and rectocele.
We presume that as there is a correlation between the anterior vaginal wall and apex, there is a correlation between the posterior vaginal wall and apex. The aim of our study is to reveal the correlation between the posterior vaginal wall and apex in POP.
Materials and methods
After Institutional Review Board approval was obtained in written form by CHA Bundang Medical Center, we retrospectively reviewed the records of consecutive new patients visit to an urogynecology clinic between January 2013 and December 2015. Each patient underwent a comprehensive urogynecologic physical examination, which included a pelvic organ prolapse quantification (POP-Q) examination [8].
POP-Q examination was performed by one practitioner with the patient in the lithotomy position during maximal Valsalva strain. Cases with previous hysterectomy, or previous POP surgery were excluded.
Data were stored and analyzed with SPSS software (version 13; SPSS Inc., Chicago, IL, USA). Pearson's correlations were used to compare individual POP-Q points when the population followed normal distribution. Spearman's correlations were used to compare individual POP-Q points when the population did not follow normal distribution. Linear regression was then used to model significant relationships between individual POP-Q points. P-values below 0.05 were considered significant.
Results
Four hundred eighty-two women met our inclusion criteria. Sixty-four patients had undergone prior hysterectomy, and 13 had prior prolapse surgery. Ultimately, four hundred five cases were enrolled in our study. Study subjects had a mean age of 65.4±11.9 years (range 29–90), mean body mass index (BMI) of 24.5±3.1 kg/m2 (range 14.7–36.1), and mean parity of 3.3±1.5 (range 0–9). There were 50 (13.3%) premenopausal and 355 (87.7) postmenopausal women (Table 1).
In total, the largest group consisted of 256 (63.2%) stage 3 POP cases; the smallest group consisted of 36 (8.9%) stage 4 cases. Posterior vaginal wall prolapse of the advanced stage (≥stage 2) occurred more frequently than apical prolapse of the advanced stage. The most common stages of Bp and C prolapses were stage 2 and stage 3, respectively (Table 2).
When all stages were included, the Bp had a moderate correlation with the C (Pearson's r=0.419; P<0.001) and when C was above stage 1, the Bp had a moderate correlation with the C (Pearson's r=0.426; P<0.001).
With this model, the correlation was more significant as stage increased. Cases where Bp was stage 2 and above presented positive moderate correlations with C (Spearman's ρ=0.523; P<0.001). Using this regression model to predict the C score based on the Bp score, one would expect the C score to be approximately 1.1 when the Bp score is 0 (Linear regression equation; C=0.359Bp+1.148; Table 3). Cases where C was stage 2 and above presented positive moderate correlations with Bp (Spearman's ρ=0.637; P<0.001). To predict the Bp score based on the C score, one would expect the Bp score to be approximately 1.6 when the C score is 0 (Linear regression equation; Bp=0.497C+1.584; Table 3).
With this model, the correlation was even more significant in stage 3 and 4 prolapse. Cases where Bp was stage 3 and above presented strong positive correlations with C (Spearman's ρ=0.783; P<0.001). To predict the C score based on the Bp score, one would expect the C score to be approximately 3.9 when the Bp score is 2 (Linear regression equation; C=0.389Bp+3.134). Cases where C was stage 3 and above presented also strong positive correlations with Bp (Spearman's ρ=0.718; P<0.001). To predict the Bp score based on the C score, one would expect the Bp score to be approximately 3.9 when the C score is 2 (Linear regression equation; Bp=0.435C+2.988; Table 3).
Discussion
The most important finding of this study was that posterior vaginal wall and apex in POP were correlated with each other and correlation was more significant as stage increased. In stage 3 or greater, apical prolapse and posterior vaginal wall prolapse is virtually always present (Spearman's ρ=0.783; P<0.001).
A gynecologist may counsel patients differently based on the location of pelvic floor weakness, such as possibly altering the actual procedure considering the information. For example, if a patient is found to have advanced stage posterior vaginal wall prolapse, the gynecologist should thoroughly evaluate accompanied apical prolapse and consider undergo repair of apical prolapse. These recommendations would be appropriate on the premise that a strong correlation exists between the posterior vaginal wall (Bp) and apex (C). Rooney et al. [6] found that the C was moderately correlated with the most prolapsed portion of the Bp (Spearman's r=0.556; P<0.001). When he included only women with stage 2 or greater, C was correlated even more strongly with the Bp (Spearman's r=0.746; P<0.001). In our study as well, the Bp and C had a moderate correlation coefficient, and the correlation coefficient was higher with an increasing stage. Although our study is somewhat similar to the study by Rooney et al. [6], but there are some differences. First, they studied the correlation between C and Bp dichotomously for all stage and stage 2 or greater. But, we analyzed correlation between C and Bp respectively by stratified stage (≥1, ≥2, ≥3). Second, the previous study evaluated correlation between C and Bp by one aspect (change of Bp point by C point as a reference point), ours evaluated correlation between C and Bp point each other. Third, we excluded subjects who had undergone hysterectomy or POP surgery to reduce bias.
Surgical failures are a concern for all gynecologists performing reconstructive pelvic surgery. The definition of surgical failure is poorly defined. Consequently, outcome analysis in surgery for POP has focused on reoperation. The gynecologists must discuss the risk of recurrence when counseling the patient for surgery. Identifying patients who may be at an increased risk for recurrent pelvic floor disorders would be helpful in such a discussion [9]. In a small cohort of women with recurrent prolapse, Clark et al. [10] found that nearly one-third of prolapse recurrences occurred at a different site from the original repair. The important cause of POP recurrence is that individual procedures may not adequately treat all vaginal compartments, which predisposes to surgical recurrence in another compartment. This is possibly because all vaginal wall defects may not be recognized before the operation, which leads to a persistent vaginal wall defect. We believe our data support the hypothesis that recurrent prolapse may be partially due to a modifiable factor, which is a failure to diagnose defects of another site, especially between the posterior vaginal wall and apex.
The strengths of our study lie in the relatively large number (405) of cases enrolled. Furthermore, all POP-Q measurements were made by a single practitioner and cases with previous hysterectomy or previous surgery were excluded, decreasing bias. The current study has several limitations. First, this is a retrospective chart review. Another major limitation involves imprecise selection of subjects. Subjects were restricted to those who with POP and were of Asian ethnicity, factors such as age and BMI were not controlled. To obtain an accurate correlation between Bp and C, subjects would need to be precisely selected to ensure reliable results.
In conclusion, posterior vaginal wall prolapse and apical prolapse were correlated with each other, and this correlation was more prominent as stage increased. Therefore, when admitting a patient suspected of posterior vaginal wall prolapse or apical prolapse, it is necessary to evaluate both conditions. Especially in cases more severe or equal to stage 3, it is a must to suspect both conditions as the 2 are strongly correlated.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.