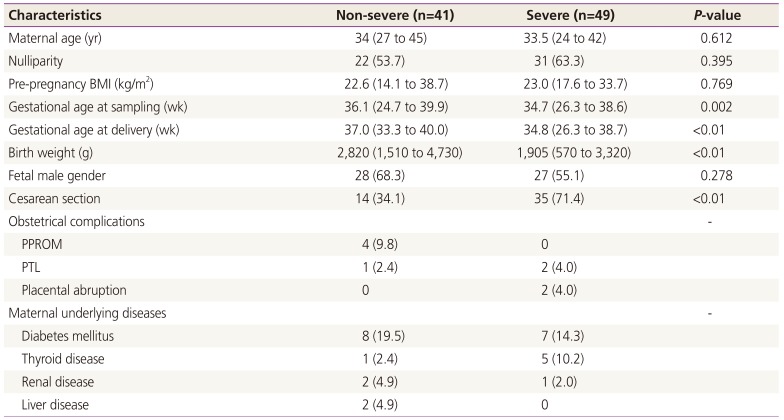
The clinical significance of D-dimer concentrations in patients with gestational hypertensive disorders according to the severity
Article information
Abstract
Objective
Pregnancy is a major risk factor of thromboembolism, and the patients with preeclampsia (PE) are known to have higher risk of thromboembolic complications than normal pregnant women. D-dimer is a well-established laboratory marker for the screening of venous thromboembolism (VTE), but the concentrations of d-dimer tend to increase physiologically in pregnant women throughout the gestational age. We performed this study to evaluate the clinical significance of d-dimer concentrations in patients with gestational hypertensive disorders (GHD) according to the severity.
Methods
Retrospective cohort study was performed in one institution. Singleton pregnant women with GHD were enrolled, and their antepartum concentrations of d-dimer were measured as a part of routine evaluation for patients suspected with PE. Patients with multiple gestations, rheumatic diseases, autoimmune diseases, or suspected VTE were excluded. A categorization of severity about PE was based on the general criteria.
Results
In 73.3% of study population, their d-dimer concentrations exceeded the normal range (>0.55 mg/L). A significantly greater proportion of pregnant women had excessive concentrations of d-dimer in the severe GHD than in the non-severe GHD (89.8% vs. 53.7%; P<0.01). Patients with severe GHD had significantly higher median concentrations of d-dimer than those with non-severe GHD (median [range], 2.00 mg/L [0.11 to 7.49] vs. 0.71 mg/L [0.09 to 5.39]; P<0.01) although their earlier gestational ages of sampling.
Conclusion
Maternal concentrations of d-dimer were significantly elevated in patients with severe features than those without severe features among those with GHD. Some pregnant women with GHD can have markedly elevated concentrations of d-dimer without any evidence of current VTE.
Introduction
Pregnancy is characterized by a hypercoagulable state due to a number of hormonal changes and alterations of clotting and fibrinolytic factors [123]. Probably, it helps to protect pregnant women from fatal hemorrhage during delivery, but it can also contribute to increase the thromboembolic risks during pregnancy and puerperium. The patients with preeclampsia (PE) are known to have higher risk of thromboembolic complications than normal pregnant women [4567]. PE is associated with deposition of fibrin in microvasculature, which results in compromised placental perfusion, fetal growth restriction (FGR) and dysfunction in some maternal organs [8910].
D-dimer is a plasma breakdown product of cross-linked fibrin and it is a widely used indicator for the exclusion of venous thromboembolism (VTE) with highly negative predictive value [11]. However, the concentrations of d-dimer in maternal blood substantially increase physiologically throughout the gestational age without any thromboembolic complication because of continuous coagulation and fibrinolysis during the normal development of the placenta [121314]. In a serial study, only 22% of women in mid-pregnancy and no women in the third trimester had a normal concentration of d-dimer (<0.50 mg/L) [15]. Increased baseline levels of d-dimer during pregnancy may be a kind of confounder in the interpretation of d-dimer results for the evaluation of thromboembolism, and d-dimer can be only useful in the limited cases with negative values during pregnancy. Previous studies suggested a higher threshold of d-dimer in pregnant women, or a gestational age-specific reference interval of d-dimer [1617]. Several studies showed that patients with PE had higher concentrations of d-dimer than normotensive controls [1819]. It may be associated with higher thromboembolic risk in patients with PE than normotensive controls.
However, there is no study to identify the concentrations of d-dimer according to the severity of gestational hypertensive disorders (GHD). We performed this study to evaluate the clinical significance of d-dimer concentrations in patients with GHD according to the disease severity.
Materials and methods
Singleton pregnant women who had GHD and deliverd at Seoul Metropolitan Government-Seoul National University Boramae Medical Center between January 2011 and August 2016 were enrolled. GHD included gestational hypertension, PE, and superimposed PE. Their antepartum concentrations of d-dimer were measured as a part of routine evaluation for patients suspected with PE. The concentrations of d-dimer were determined by immunologic assay (Sysmex® CA-7000, from January 2011 to December 2015 and Sysmex® CS-5100 since January 2016; Siemens Healthcare GmbH, Erlangen, Germany). The instruments had clinical cut-off of 0.55 mg/L in human plasma. The concentration of d-dimer above 0.55 mg/L was reported as abnormal value in our institution. Patients without d-dimer levels or those who had underlying risks of elevated d-dimer concentrations, for example, multiple gestations, rheumatic diseases, autoimmune diseases, or suspected thromboembolism were excluded. D-dimer was one of the components included in the disseminated intravascular coagulation (DIC) panel in our institution, and clinicians did not consider the concentrations of d-dimer clinically in the diagnosis or management of patients with GHD.
Gestational hypertension was defined as new-onset hypertension (systolic blood pressure [BP] ≥140 mmHg and/or diastolic pressure ≥90 mmHg on 2 occasions at least 4 hours but no more than 7 days apart) after 20 weeks of gestation in a previously normotensive woman. If the women met one of the following criteria, they were classified as patients with PE: 1) significant proteinuria (≥300 mg/24 h or protein/creatinine ratio ≥0.3 or ≥1+ on dipstick), 2) thrombocytopenia (platelet <100,000/µL), 3) renal insufficiency (creatinine >1.1 mg/dL or doubling of baseline), 4) liver involvement (serum transaminase levels twice normal or upper abdominal pain), 5) cerebral symptoms (headache, visual disturbance, convulsions), and 6) pulmonary edema. Superimposed PE was diagnosed when worsening hypertension or findings associated with PE appeared in women with chronic hypertension. A categorization of severity about PE was based on the presence of one or more of the following criteria: persistent systolic BP ≥160 mmHg or diastolic BP ≥110 mmHg (BP still high after resting, which was required for anti-hypertensive medication), headache, visual disturbances, upper abdominal pain, oliguria, convulsion (eclampsia), elevated serum creatinine (>1.1 mg/dL), thrombocytopenia (<100,000/µL), marked serum transaminase elevation (more than twice the normal range), obvious FGR or pulmonary edema [20]. There are various causes of FGR other than placental insufficiency, so we defined obvious FGR by GHD as FGR below 10th percentile for gestational age, and accompanied with abnormal umbilical artery Doppler findings (absence or reverse of end-diastolic flow) or oligohydramnios (amniotic fluid index <5) on ultrasound scan. Pulmonary edema was diagnosed by respiratory symptoms and chest radiographs. We defined severe GHD as GHD with one or more severe features as above, and non-severe GHD as GHD without severe features.
Statistical analysis was carried out using International Business Machines (IBM) Statistical Package for the Social Sciences (SPSS) statistics software ver. 20.0 (IBM Corp., Armonk, NY, USA). Nonparametric techniques were used for statistical analysis. Comparison of the continuous variables was performed using the Mann-Whitney U test, and proportions were compared using Pearson χ2 test. Logistic regression analysis was used to examine the levels of d-dimer according to severity after adjustment for the effect of gestational age at sampling. A probability value of <0.05 was considered statistically significant. The receiver operating characteristic (ROC) curve analysis was performed to assess the cut-off value of d-dimer level to identify severe GHD.
This study was approved by the Institutional Review Board (IRB) of Seoul Metropolitan Government-Seoul National University Boramae Medical Center (IRB No. 16-2017-60).
Results
Of the 90 pregnant women who met the inclusion criteria, 49 had severe GHD including severe PE or superimposed PE, and 41 had non-severe GHD. Demographic and clinical characteristics of the study population are summarized in Table 1. There were no statistical differences between the 2 groups in terms of maternal age, parity, or pre-pregnancy BMI. However, women with severe GHD has significantly earlier median gestational age at sampling, earlier median gestational age at delivery, lower median birth weight, and higher cesarean section rate compared with those with non-severe GHD. These differences may be attributed to the characteristics of severe PE.

Clinical characteristics of the study population according to the severity of gestational hypertensive disorders
In 73.3% of total study population, the concentrations of d-dimer exceeded the normal range (>0.55 mg/L). A significantly greater proportion of pregnant women had excessive concentrations of d-dimer in the group of severe GHD than non-severe GHD (89.8% vs. 53.7%; P<0.01). Patients with severe GHD had significantly higher median concentrations of d-dimer than those with non-severe GHD (median [range], 2.00 mg/L [0.11 to 7.49] vs. 0.71 mg/L [0.09 to 5.39]; P<0.01) despite their earlier gestational age of sampling (Table 2). The difference was significant after adjusting for gestational age at sampling. Fig. 1 showed that concentrations of d-dimer according to the severity indicators of GHD. A number of patients had two or more severe features, and persistent high blood pressure (BP) was the most common feature. All of 3 patients with pulmonary edema and only 1 case with thrombocytopenia had quite high d-dimer levels (2.09, 4.58, 5.48, and 7.40 mg/L). The median concentration of d-dimer in patients with pulmonary edema was higher than in those with high BP, but that could not reach statistical significance (P=0.09). There was no case with eclampsia in our study population. Table 3 demonstrated clinical characteristics of patients with the highest 10th percentile concentrations of d-dimer. All of them had markedly elevated concentrations of d-dimer over 4.00 mg/L. Most of patients (7/9) had severe GHD and delivered at preterm. However, two of them had non-severe GHD and delivered at term without complications until puerperal period despite of their elevated level of d-dimer. Using ROC curve analysis, a cut-off value of 1.19 mg/L (ROC area under the curve, 0.71; 95% confidence interval, 0.60 to 0.82; P=0.001) for maternal concentration of d-dimer had 63.3% of sensitivity and 65.9% of specificity for the identification of severe GHD (Fig. 2).
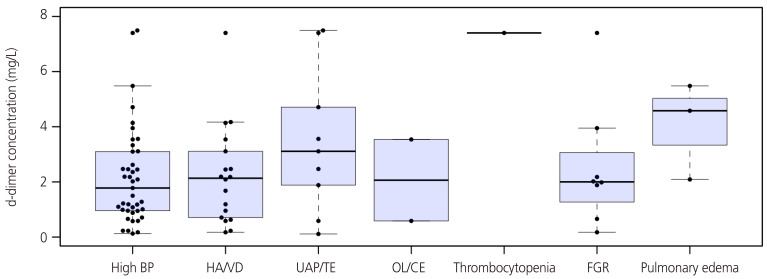
Concentrations of D-dimer according to the severity indicators of gestational hypertensive disorders. The median concentration of d-dimer in patients with pulmonary edema was higher than in those with high blood pressure, but that could not reach statistical significance (P=0.09).
BP, blood pressure; HA/VD, headache or visual disturbance; UAP/TE, upper abdominal pain or serum transaminase elevation; OL/CE, oliguria or elevated serum creatinine elevation; FGR, fetal growth restriction.

Receiver operating characteristic (ROC) curve analysis for identification of severe gestational hypertensive disorder. Using ROC curve analysis, a cut-off value of 1.19 mg/L (ROC area under the curve, 0.71; 95% confidence interval, 0.60 to 0.82; P=0.001) for maternal concentration of d-dimer had 63.3% of sensitivity and 65.9% of specificity for the identification of severe gestational hypertensive disorders.
Discussion
Our data demonstrated that about 80% of patients with GHD had excessive concentrations of d-dimer above normal limits. The median concentration of d-dimer in maternal plasma was significantly higher in patients of severe GHD than those of non-severe GHD among those with GHD. Considering substantial increase of d-dimer concentrations throughout gestational age [12142122], this difference is more evident because the gestational age of the severe GHD was significantly earlier than that of non-severe GHD. Our data represented that about 80% of patients with the highest 10th percentile concentrations of d-dimer had severe GHD and delivered at preterm. However, there were a few cases with non-severe GHD and full-term delivery even though their markedly elevated levels of d-dimer. Among the patients with severe features, those with pulmonary edema showed a moderate trend for higher levels of d-dimer than those with high BP, and a patient with thrombocytopenia had markedly elevated level of d-dimer. And in our data, the sensitivity and specificity of d-dimer value for the identification of severe PE by ROC curve analysis were relatively low. Although the difference of median concentration of d-dimer between the 2 groups was statistically significant, pregnant women with GHD had various range of d-dimer concentrations and the overlapping range was rather wide. Therefore, it may be difficult to use the d-dimer as screening tool for the identification of severe GHD.
D-dimer represents the ultimate degradation product of fibrin by plasmin and its presence in plasma is an indirect activation of coagulation and fibrinolysis [23]. The concentrations of d-dimer reflect both fibrin polymerization and its breakdown in vivo [242526], and the high concentrations in patients with severe PE are probably due to fibrin production. This correlates the concept of pathophysiology of PE that microthrombus formation and excess fibrin deposition affecting multiple maternal organs as well as the placenta resulting in hypoperfusion [27]. Pregnant women with PE are known to have higher hypercoagulable state, as well as inflammatory response compared to those with normotensive controls. Recently, PE has been associated with 3 to 7 fold increased risk of pregnancy associated VTE [4567]. However, precise molecular mechanisms underlying this clinical observation have yet to be determined. In previous studies with non-pregnant general papulation or elderly patients, higher d-dimer levels without current VTE were associated with risks of subsequent development of VTE [2829]. According to these studies, even in the absence of clinically overt venous thrombosis, the determination of d-dimer level is likely to be helpful for the identification of patients at risk of subsequent VTE. However, we failed to assess the value of d-dimer for the prediction of VTE because there was no case with postpartum VTE in our small number of study population. Actually, the risk of VTE and level of d-dimer is much higher in postpartum state than during pregnancy.
A limitation of this study was that the level of d-dimer was measured only in patients with hypertensive disorders during pregnancy for evaluation of PE. We have no reference data of d-dimer levels in normal contemporary pregnant women according to the gestational age. Also, the study population consisted of a small number of patients because it was a retrospective cohort study. Only some patients among those with GHD have the results of d-dimer concentrations because d-dimer is one of the components in ‘DIC panel,’ but not included in ‘Coagulation panel’. Clinicians chose either a ‘DIC panel’ or ‘Coagulation panel’ as a part of evaluation of PE. If we can perform serial measurements of d-dimer levels in all pregnant women with GHD and normotensive controls according to the gestationaly age in further prospective study, that will be helpful to investigate the various implications of elevated levels of d-dimer in pregnant women, especially association with subsequent venous thromboemboilsm at postpartum. There was no case with postpartum VTE in our small number of study population.
In conclusion, pregnant women with severe GHD tend to have higher concentrations of d-dimer than those without severe feature among those with GHD. Especially, in patients with pulmonary edema or thrombocytopenia, the levels of d-dimer were elevated in all cases. Some of patients with GHD can have marked elevated concentrations of d-dimer without any evidence of current thromboembolic complications.
Acknowledgements
The authors would like to thank Sohee Oh, PhD of the Department of Biostatistics in Seoul Metropolitan Government-Seoul National University Boramae Medical Center for statistical advice.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.