Efficacy and safety of venous thromboembolism prophylaxis with fondaparinux in women at risk after cesarean section
Article information
Abstract
Objectives
Cesarean section is associated with an increased risk for venous thromboembolism (VTE). The safety and efficacy of primary prophylaxis of fondaparinux, a synthetic sulfated pentasaccharide heparin analog, in women at risk after cesarean section is uncertain.
Methods
This was a retrospective study of 295 cases of pregnant women presenting to a tertiary referral center of Nara, Japan, to evaluate the usefulness of thromboprophylaxis with fondaparinux after cesarean delivery between 2011 and 2012. Patients were initially received unfractionated heparin (once 5,000 IU subcutaneously, twice a day), starting 6 hours after cesarean section for 24 hours, and then treated with fondaparinux (once 2.5 mg daily, subcutaneously) for 5 days. The primary efficacy end-point was an improvement in the incidence of symptomatic VTE or fatal post-cesarean pulmonary thromboembolism. The primary safety end-point was major bleeding during treatment.
Results
There were neither any episodes of symptomatic VTE cases nor maternal deaths. A total of 10 patients had a bleeding event. Major bleeding complication was observed in 2 (0.68%) of 295 patients receiving fondaparinux. Non-major bleeding into critical sites was observed in 8 patients, often at surgical sites, and recovery was not delayed.
Conclusion
This study demonstrates the safety and efficacy of fondaparinux in women at high risk of VTE after cesarean section. Large phase trials comparing clinical outcomes with fondaparinux across a wide spectrum of patients are needed to confirm these observations.
Introduction
Major surgery, involving the pelvis or lower limb, conveys an increased risk of venous thromboembolism (VTE), comprising a spectrum of thromboembolic disease including both deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE) [1]. The incidence of VTE in young women is approximately 2 persons per 10,000 per year [2]. Overall the incidence is 6–18 VTEs per 10,000 deliveries [3]. Pregnancy increases this risk approximately 5 times [3]. The VTE rate after cesarean delivery is 0.5%, demonstrating that women after cesarean section remain at high risk for VTE events [4].
Antithrombotic treatment and prophylaxis both before and during pregnancy are based on unfractionated heparin (UFH), low-molecular weight heparin (LMWH), and aspirin [5]. Antenatal LMWH was associated with fewer adverse effects when compared with UFH [6]. Therefore, LMWH is considered to be a safe and effective treatment for the majority of cases of pregnancy-related VTE [3]. In the American College of Chest Physicians evidence-based clinical practice guidelines (8th and 9th edition), pregnant women with a single prior episode of VTE, a higher risk thrombophilia or pregnancy complications, who undergoing cesarean section, are recommended to receive both mechanical and anticoagulant prophylaxis with LMWH [78].
Unfortunately, the use of thromboprophylaxis after cesarean surgery is not routine in Japan. Practice guideline recommendations for the prevention of VTE in these patients have not been fully implemented [9]. This is partly owing to concerns about bleeding risks and the safety during breast-feeding. This educational tertiary referral hospital established a policy with fondaparinux (Arixtra®) as postoperative thromboprophylaxis (gynecological, obstetrical, abdominal, and orthopedic surgery) in 2011. Fondaparinux is a completely synthetic pentasaccharide heparin analog and the first of a new class of selective indirect antithrombin-dependent factor Xa inhibitors, which inhibits thrombin generation. The advantages of fondaparinux is the lack of need for routine laboratory monitoring and once daily fixed dosing. This anticoagulant has been widely used in clinical practice for patients undergoing orthopedic surgery and the periprocedural bleeding is low [10]. In Japan, patients were sometimes treated with 1.5 mg of fondaparinux instead of 2.5 mg of fondaparinux due to adverse effects including an increased incidence of subcutaneous knee hematoma [11].
Although there exists a concern about the use of LMWH in pregnant women, there is no evidence to support fondaparinux as an alternative to LMWH. The safety and optimal use of fondaparinux in high risk women after cesarean section remains uncertain since these pregnant women were excluded from clinical trials. The aim of this study was to explore the efficacy and safety of post-cesarean thromboprophylaxis with fondaparinux.
Materials and methods
1. Patient selection and study procedures
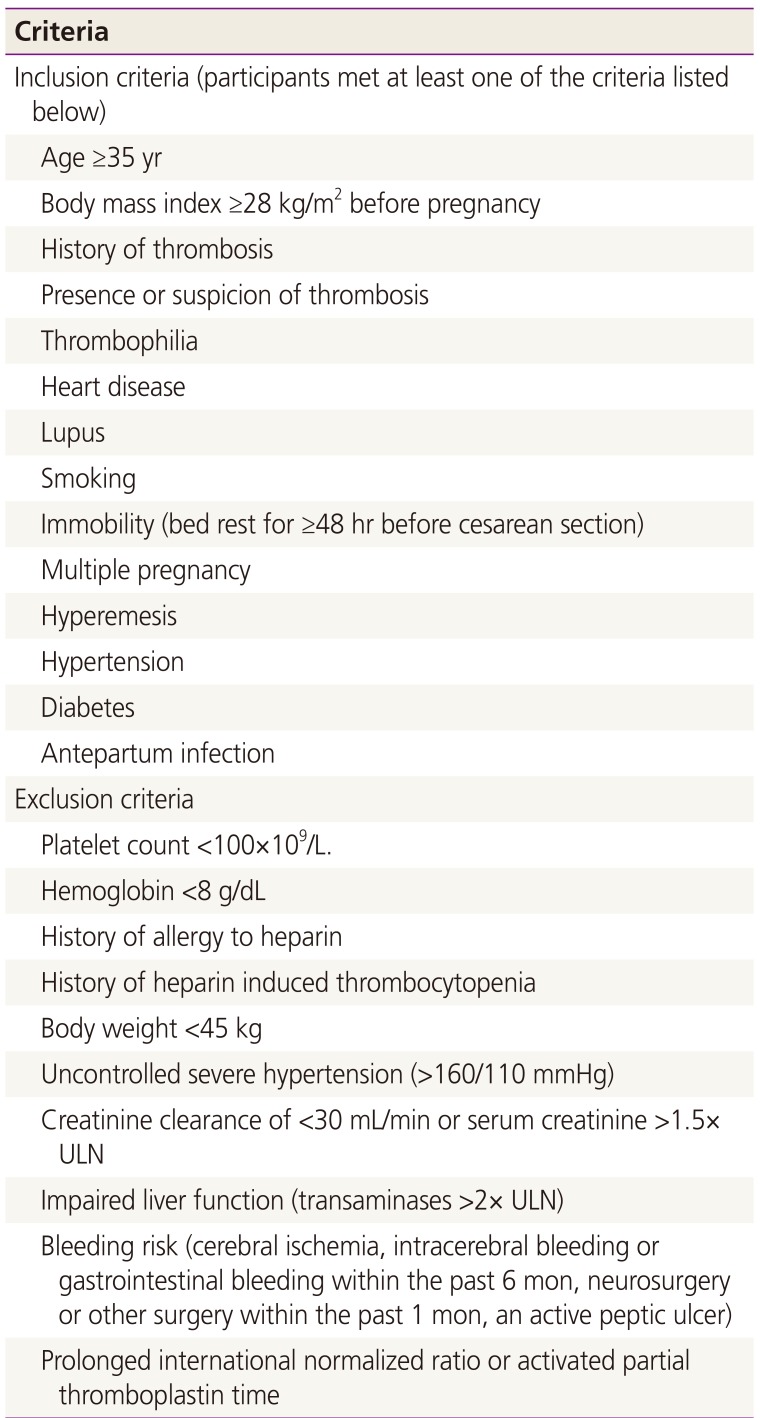
This was a retrospective study of 295 cases of pregnant women presenting to a tertiary referral center of Nara, Japan, to evaluate the usefulness of thromboprophylaxis with fondaparinux after cesarean delivery. From August 1, 2011 to December 31, 2012, in our hospital 1,125 deliveries were attended. During the period of this study, 422 women who underwent cesarean section were considered eligible if they met at least one of the following criteria (Table 1): 35 years or older, obesity ≥28 of body mass index, a history of thrombosis, thrombophilia, heart disease, lupus, smoking, immobility (bed rest for ≥48 hours before cesarean section), and obstetric complications including multiple pregnancy, hyperemesis, hypertension, diabetes, and postpartum infection [12]. The study was approved by Institutional Review Boards (Nara Medical University; authorization number 06-002).
The main exclusion criteria (Table 1) were related to bleeding risk: cerebral ischemia; intracerebral bleeding or gastrointestinal bleeding within the past 6 months; neurosurgery or other surgery within the past 1 month; an active peptic ulcer; a known bleeding disorder; prolonged international normalized ratio or activated partial thromboplastin time; a platelet count <100×109/L and hemoglobin <8 g/dL. Other exclusion criteria included body weight <45 kg; uncontrolled severe hypertension (>160/110 mmHg); creatinine clearance of <30 mL/min or serum creatinine >1.5× upper limit of normal (ULN); and impaired liver function (transaminases >2× ULN).
The number of VTE events, time to VTE event, length of stay (LOS) in hospital, and number of major or minor bleeding (non-major bleeding) events were analyzed from the index date until the end of follow-up (3 months post discharge) or death. Development of VTE was assessed by a direct interview, a clinical examination in out-patient clinic, and phone calls to patients for history taking, if needed, using a questionnaire-based interview format. The interview includes open questions, and each woman is asked about their symptoms of VTE by answering the questionnaire. Clinical follow-up of patients continued until three months after the last dose of study medication.
2. Administration of fondaparinux
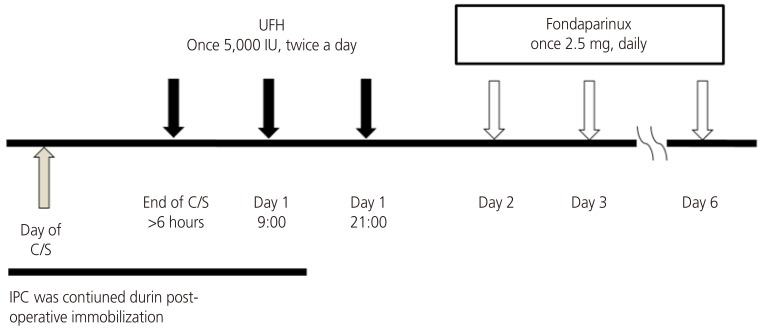
Japanese guideline for prevention of VTE has recommended that to minimize bleeding complications, administration of fondaparinux should be postponed until 24 hours after cesarean delivery (http://www.medicalfront.biz/html/06_books/01_guideline/). Women initially received UFH (once 5,000 IU subcutaneously, twice a day), starting 6 hours after cesarean surgery for 24 hours, and then treated with fondaparinux (once 2.5 mg daily, subcutaneously) for 5 days (Fig. 1). Graduated compression elastic stockings and/or intermittent pneumatic compression devices were applied intraoperatively and continued during post-operative immobilization.
3. Laboratory assessments
Blood samples were collected from each patient for analysis of clinical chemistry, blood cell counts, and blood coagulation at the time of being included in the study (before surgery) and on treatment days 1, 3, and 6. In patients whose laboratory test results such as liver and renal function tests became abnormal (confirmed by 2 consecutive readings), the decision to discontinue fondaparinux therapy or continue under close laboratory supervision was based on a predetermined algorithm.
4. Efficacy and safety outcomes
The efficacy outcome variables were objectively confirmed symptomatic VTE or VTE-related death up to 3 months following cesarean. Ultrasonography examination, high probability ventilation perfusion lung scanning and/or spiral computerized tomography and/or angiography were performed only in patients with clinical suspicion of thrombosis or embolism. All sonographers received medical training to ensure a high quality of standardized ultrasonography. Study treatment will be stopped upon confirmation of symptomatic PTE or major bleeding events, which was managed according to usual care.
The primary safety outcome was the incidence of major bleeding with onset no later than 1 day after the last dose of fondaparinux. Major bleeding is defined as a symptomatic bleeding complication: Bleeding was considered major if it was fatal, affected a critical organ with symptomatic hemorrhage (retroperitoneal, intraabdominal, intracranial, intraocular, or intraarticular), or was clinically overt bleeding warranting treatment cessation, a fall in blood hemoglobin ≥2 g/dL, and required a transfusion of 2 or more units of blood [13]. Secondary safety measures include the incidence of non-major bleeding events. Non-major bleeding was considered to be any other bleeding not meeting the definition for major bleeding. The medical records of these patients were analyzed retrospectively for the following variables: patient demographics, diagnosis and comorbidities, operative and anesthetic details, postoperative VTE prophylaxis, postoperative complications including bleeding, and hospital readmission within three months of surgery.
Results
1. Patient characteristics
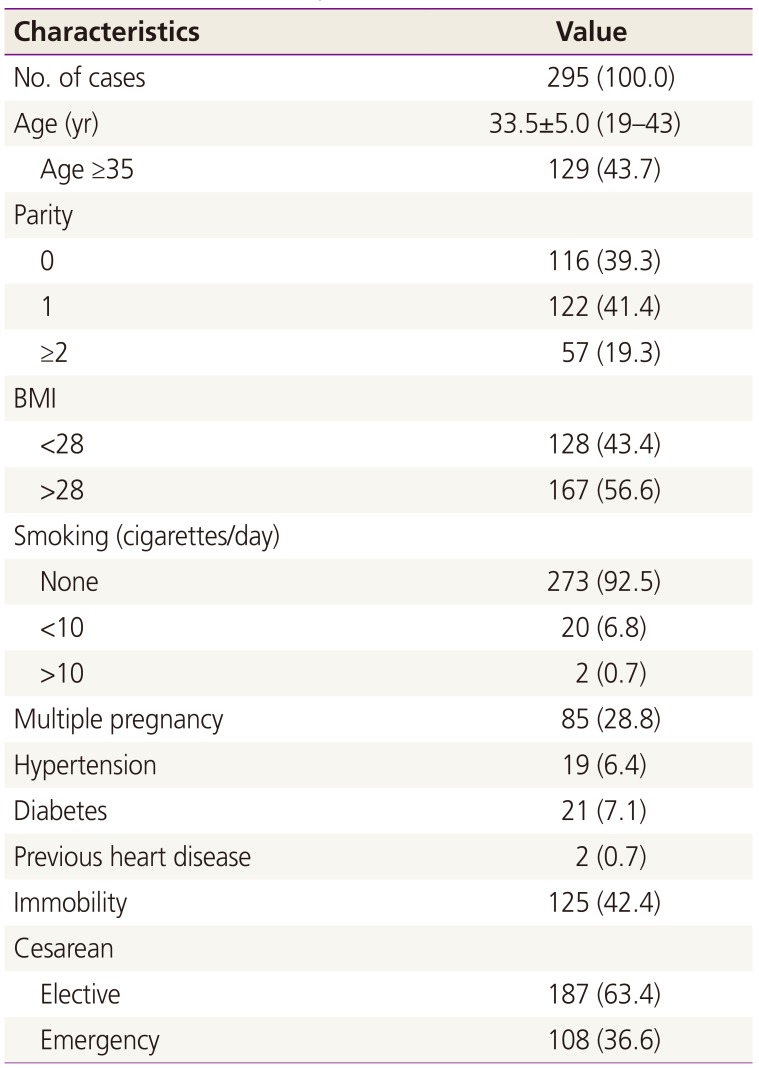
Two hundred ninety-five were analyzed. Table 2 shows the characteristics of the patients. The mean age of the patients was 33.5±5.0 years (range, 18–42). The median LOS in hospital was 7 days (range, 6–15). Spinal anesthesia and general anesthesia was used in 95.3% (n=281) and 4.3% (n=14) of patients, respectively. The mean operative duration was 54.0±15.5 minutes (range, 25–93). All of the present series of women with cesarean had at least one more risk factor. The median number of comorbidities was 2 (range, 1–4); obesity (n=167), 35 years or older (n=129), immobilization due to pregnancy-related conditions (n=125), multiple pregnancy (n=85), smoking (n=22), hypertension (n=19), placental abruption (n=13), and heart disease (n=2).
2. Efficacy outcomes
All patients were available for follow-up. No symptomatic VTE events occurred in hospital. The patients were followed up for 3 months after surgery by physical examination in out-patient clinic and the structured clinical interview to determine the incidence of symptomatic VTE. From the time of original admission to 3 months after discharge, the overall incidence of symptomatic VTE was 0%.
3. Safety outcomes
A total of 10 patients had a bleeding event. Major bleeding complication was observed in 2 (0.68%) of 295 patients receiving fondaparinux. The 2 cases suffered from intraabdominal hematoma, which could be managed conservatively. Non-major bleeds into critical sites were observed in 8 patients, often at surgical sites (wound hematoma, 3 cases [1.02%]; hematoma around the uterine incision, 2 cases [0.68%]; or other signs of superficial bleeding, 3 cases [1.02%]), and recovery was not delayed. Four (50.0%) of 8 patients with non-major bleeding complications stopped taking the drug.
4. Other observations
Two women receiving fondaparinux every 24 hours developed hepatotoxicity on the day 6 after the initiation of treatment. The results of laboratory tests revealed elevated levels of hepatic enzymes, including alanine transaminase (ALT) and aspartate transaminase (AST). Twenty patients (6.8%) showed an abnormality of ALT or AST on the day 6 of drug initiation. Aminotransferase levels returned to below the ULN within 14 days.
Discussion
To our knowledge, this is the first study to evaluate the safety and efficacy of a once-daily, fixed-dose regimen of fondaparinux for the thromboprophylaxis of women at risk after cesarean section. During the study treatment period, none of the in-patient and post-discharge women developed symptomatic VTE. There were two cases of major bleeding (0.68%) and eight clinically relevant non-major bleeding (2.7%). Fondaparinux may be particularly promising as a prophylactic drug.
It has been reported that the total incidence of VTE was 1.72 per 1,000 deliveries [14]. The odds ratio for cesarean versus vaginal delivery was 2.1. Given the total number of deliveries at the hospital during this period, the expected number of newly-developed VTE patients could be 3 to 4 cases related to 1,000 cesarean deliveries based on the previous model-based incidence rates [14]. Cavazza et al. [15] assessed the efficacy of thromboprophylaxis following cesarean delivery. The incidence of DVT was 0.2% in women at moderate-high risk who received pharmacological LMWH (enoxaparin) prophylaxis. The fact that no case was observed in our population may suggest a lower-than-expected VTE rate, indicating that fondaparinux thromboprophylaxis can reduce thromboembolism risk. Many pregnant women in our study have risk factors for VTE (obesity, 56.6%; emergency cesarean section, 53.2%; age over 35 years, 43.7%; immobility, 42.4%; multiple gestation, 28.8%; hypertension, 6.4%; diabetes, 2.7%; smoking, 1.0%; and previous heart disease, 0.7%). Our study is in contrast with other studies, where post-discharge VTE is a more common complication in the patients undergoing cesarean section who received enoxaparin prophylaxis [1516]. However, since we focused on clinically symptomatic VTE complications, we may have missed the majority of asymptomatic cases with calf vein thrombosis that may be clinically less important. The limitations are that the sample size is not sufficiently large to exclude a clinically relevant thromboembolic risk.
Fondaparinux may carry concerns regarding potential harm, including an increased risk for bleeding and hepatotoxicity. In this study, bleeding complications occurred in 3.4% of patients; all bleeding events including surgical-site bleeding were clinically judged as major (0.68%) and non-major (2.7%) bleedings. These data allow us to speculate that fondaparinux showed a clinically important reduction in symptomatic VTE and major bleeding events were uncommon. Twenty patients (6.8%) showed an abnormality of liver damage markers on the day 6 of drug initiation. Similar to fondaparinux, transient elevations in hepatic transaminase levels were noted in small percentages of patients in LMWH groups [1718].
Fondaparinux might be the drug of choice to prevent VTE in Japanese women at risk after cesarean section, but the optimal dose for prevention while avoiding bleeding is unclear. This dose was selected for the present study after considering the recommendation of Evidence-Based Clinical Practice Guidelines in major orthopedic surgery, in which fondaparinux 2.5 mg/day appeared to be the optimal regimen [19]. Additional trials and postmarketing surveillance will be needed to demonstrate proof-of-principle including the doses and the treatment schedule of fondaparinux for the prevention of VTE after cesarean delivery.
Notwithstanding these limitations, fondaparinux has some benefit for thromboprophylaxis. Heparin-induced thrombocytopenia (HIT) is an extremely rare situations of fondaparinux in comparison with the UFH and LMWH, suggesting that fondaparinux is an alternative for the treatment of thrombosis associated with HIT [2021]. Ciurzyński et al. [22] represented the first reported patient describing successful clinical applications of fondaparinux in the treatment of HIT in a third-trimester pregnant woman. There are some reports of fondaparinux being used in this situation in pregnancy but data in pregnant women are too limited to recommend fondaparinux [823].
In conclusion, short term fondaparinux appears to be an adequate and safe method for prevention of symptomatic VTE in women at risk after cesarean section. However, 2 cases of major were reported. Limitations of our study include the sample size was too small. So, large phase trials comparing clinical outcomes with fondaparinux across a wide spectrum of patients are needed to confirm these observations.
Acknowledgements
We thank all the study participants, Toshiyuki Sado, Katsuhiko Naruse, Hiroshi Shigetomi, Taihei Tsunemi, Juria Akasaka, Aiko Shigemitsu, for their time and efforts.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.