|
 |
- Search
| Obstet Gynecol Sci > Volume 59(1); 2016 > Article |
Abstract
Objective
To compare the efficacy of different add-back regimens on hypoestrogenic symptoms during postoperative gonadotropin-releasing hormone (GnRH) agonist treatment in endometriosis patients.
Methods
This prospective cohort study included reproductive-aged women who underwent conservative laparoscopic surgery for ovarian endometriosis and received add-back therapy during a 6-month course of GnRH agonist therapy after surgery. Participants received one of four different add-back regimens: 1 mg of estradiol valerate, 2.5 mg of tibolone, or a combination of 1 mg of estradiol and 2 mg of drospirenone or 0.5 mg of norethisterone acetate. Changes in quality of life, hypoestrogenic symptoms, and bone mineral density were compared according to add-back regimens.
Results
A total of 57 participants completed a 6-month course of GnRH agonist and add-back therapy. All components of quality of life did not differ across groups. However, within the same treatment group, social relationship factors decreased significantly with estradiol valerate and tibolone alone, and environmental factors decreased significantly with estradiol valerate alone. Menopausal Rating Scale score did not change significantly, but the incidence of hot flushes significantly decreased with a combination of estradiol and norethisterone acetate. Bone mineral densities at the lumbar spine declined significantly after treatment in all groups except with a combination of estradiol and norethisterone acetate.
Endometriosis affects approximately 10% of reproductive-age women and is the second most common gynecologic disease after uterine fibroids [1]. Among various treatment strategies, gonadotropin-releasing hormone (GnRH) agonists are a well-established and effective method for preventing recurrence or managing pain symptoms [2,3]. However, GnRH agonist treatment can cause problems related to prolonged hypoestrogenism, such as climacteric symptoms (hot flush, sweating, and vaginal dryness) or bone loss of up to 13% over 6 months [4,5]. These symptoms may affect the quality of life in terms of health, work productivity, and daily life activities [6,7,8]. Psychological problems that are associated with endometriosis treatment, such as anxiety and depression, can also affect quality of life [9,10]. To minimize or eliminate the hypoestrogenic side effects of GnRH agonists for endometriosis treatment, add-back therapy has been recommended [11,12,13]. To our knowledge, comparative data on the effects of add-back therapy have been still limited, although various add-back therapy regimens have been used. In addition, no previous study has compared the impact of various add-back regimens on quality of life.
This study was conducted to compare the efficacy of various add-back regimens on hypoestrogenic problems related to GnRH agonist treatment.
This study included patients who underwent conservative laparoscopic surgery for ovarian endometrioma in the Endometriosis Clinic of Samsung Medical Center in Seoul, Korea, between February 2012 and June 2013. The inclusion criteria were as follows: (1) patients with endometriosis confirmed by histology, (2) patients who planned to be treated with a 6 month course of GnRH agonist after surgery, and (3) patients who had the ability to complete a questionnaire and communicate clearly. Patients who stopped GnRH agonist therapy before completing the 6-month course and patients who did not agree to participate in the study were excluded. This study protocol was approved by the institutional review board, and informed consent was obtained from all participants.
GnRH agonist (leuprorelin acetate 3.75 mg; Leuprin, Takeda, Osaka, Japan) was administered on the day that the pathologic diagnosis was confirmed and then every four weeks for a total of six cycles. To minimize side effects of GnRH agonist injections, patients received one of the four following daily add-back regimens on the same day of GnRH agonist injection in order: 1 mg of estradiol valerate alone (Progynova, Bayer-Shering Pharma, Berlin, Germany); 2.5 mg of tibolone (Livial, MSD, Kenilworth, NJ, USA); 1 mg of estradiol combined with 2 mg of drospirenone (Angeliq, Bayer-Shering Pharma); or 1 mg of estradiol combined with 0.5 mg of norethisterone acetate (Cliovelle, DR.KADE Pharma, Berlin, Germany).
To assess the efficacy of add-back therapy, quality of life and menopause rating scales were measured three times: just before surgery, at the third injection, and one month after the sixth injection of GnRH agonist. On each visit, face-to-face interviews were performed by the same doctor.
Quality of life was evaluated using the World Health Organization Quality of Life Questionnaire, which contains 24 questions in four domains: physical health (7 items), psychological health (6 items), social relations (3 items), and environment (8 items). This questionnaire is composed of a Likert-type scale from 1 to 5 (1 for lowest agreement and 5 for highest agreement). A mean estimate for all items in each domain was transformed to the range 0 to 100.
Hypoestrogenic symptoms induced by GnRH agonist were assessed by the Menopause Rating Scale (MRS), which includes 11 questions rated on a 5-point scale (from 0 for "no symptoms" to 4 for "very severe"). The total MRS score ranges from 0 to 44, and higher scores reflect severely perceived menopausal symptoms [14].
Bone mineral density (BMD) was measured at the lumbar spine (L1-4) and total hip using dual-energy X-ray absorptiometry (Delphi Q, Hologic Inc., Bedford, MA, USA) before and after completion of the 6-month course of GnRH agonist therapy. The in vivo coefficient of variation in our center was 1.3% for the lumbar spine and 1.4% for the hip. To assess tolerability, patients were asked about side effects, including irregular uterine bleeding, on every visit. Statistical analyses were performed with IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). Data are expressed as mean±standard deviation or number (%). The Mann-Whitney or Kruskal-Walis tests were used to compare continuous variables, and Fisher' exact or chi-square tests were used to compare categorical data as indicated. Serial changes in quality of life and MRS were tested using repeated measures analysis of variance after tests for normality. P -values of less than 0.05 were considered statistically significant.
Among the 71 patients initially included in the study, a total of 57 completed 6 months of GnRH agonist treatment; 9 patients were lost to follow-up and 5 patients stopped GnRH agonist treatment before completing 6 cycles. Finally, 16 patients were in the estradiol valerate alone, 14 were in the tibolone, 13 were in the combination of 1 mg estradiol and 2 mg drospirenone, and 14 were in the combination of 1 mg estradiol and 0.5 mg norethisterone acetate group.
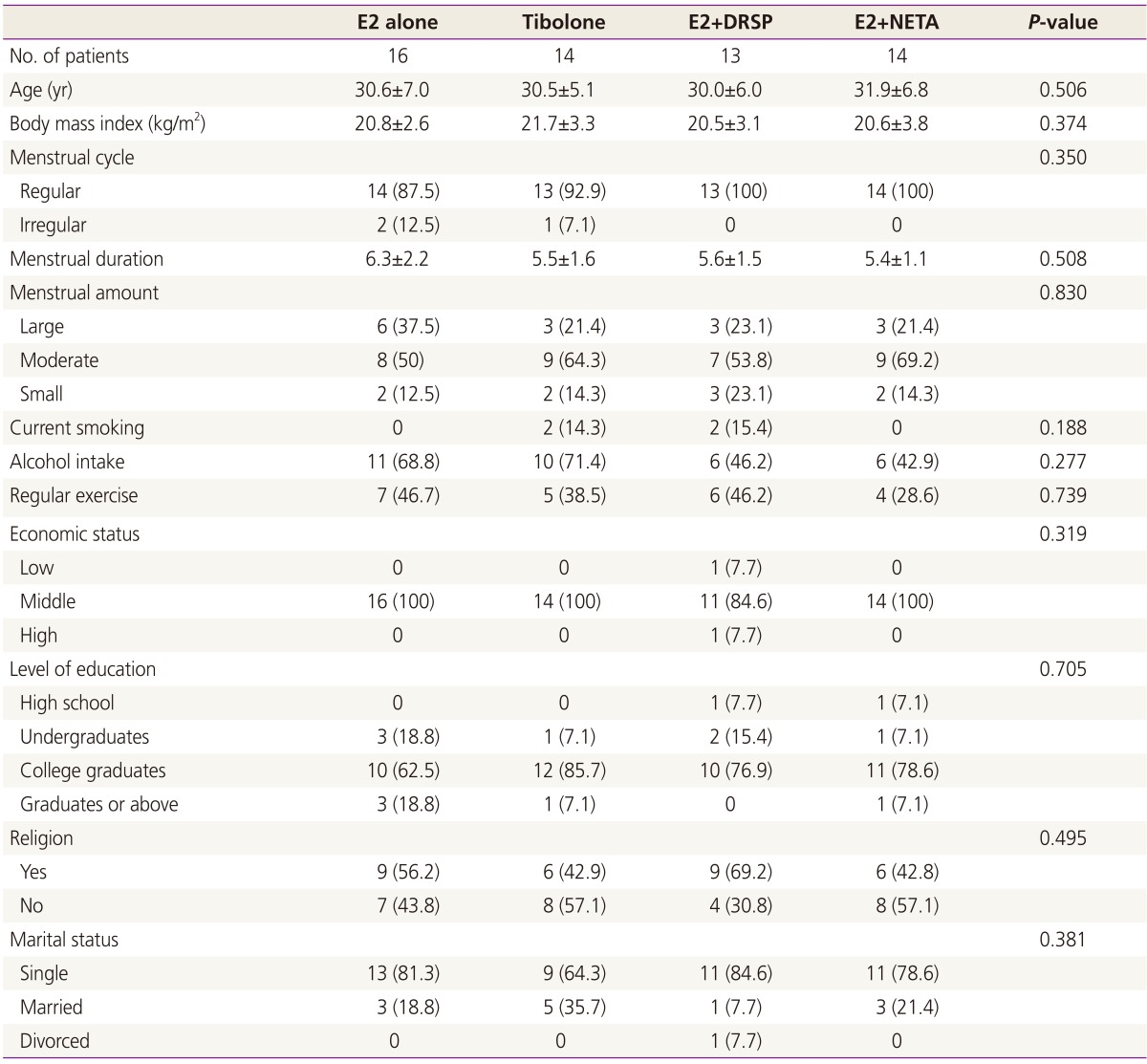
Clinical characteristics of each group are shown in Table 1. There were no differences in age, body mass index, or medical history. In addition, factors associated with quality of life, such as menstrual pattern or socio-economic status (economic status, education, religion, or marital status), also did not differ among groups.
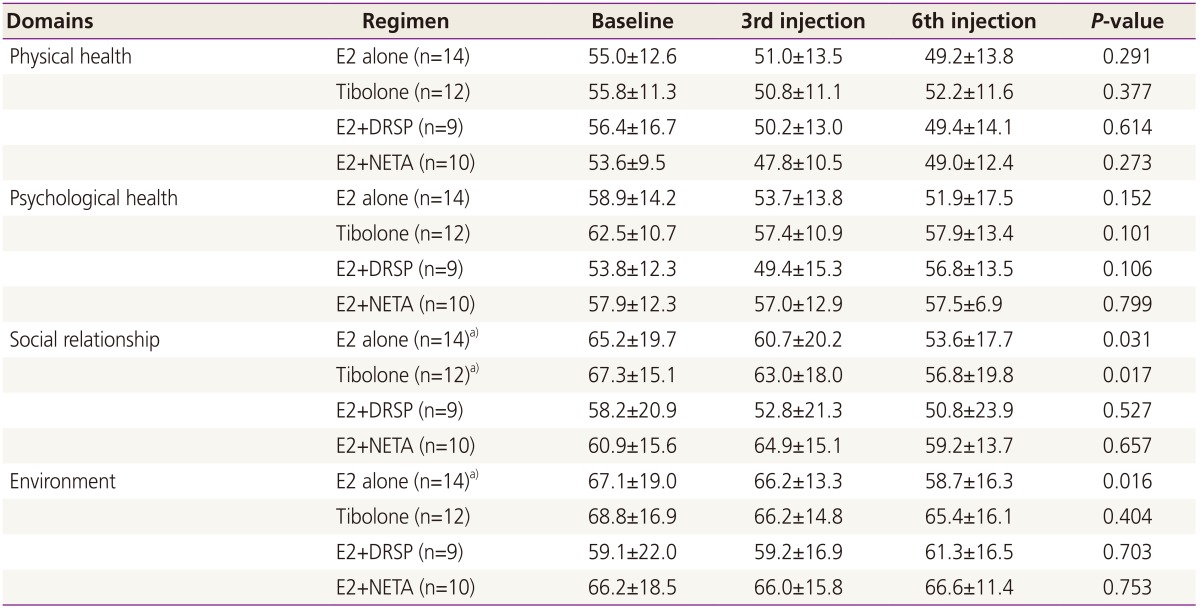
Table 2 presents changes in quality of life according to various add-back regimens. Before surgery, no difference was found in all domains across the four groups. With add-back therapies, scores of physical and psychological health did not change significantly within or between treatment groups. However, social relationship scores in the estradiol valerate and tibolone groups decreased significantly, as did environment scores in the estradiol valerate group.
In addition, MRS scores did not change significantly within or between different add-back regimens. Among the many questions included in the MRS scale, hot flush is considered to be the main representative symptom related to hypoestrogenic states caused by GnRH agonist treatment: although the incidence of hot flush decreased with all regimens, only the regimen containing 1 mg of estradiol and 0.5 mg of norethisterone acetate reduced hot flush in a statistically significant manner (data not shown).
Fig. 1 depicts changes in BMD. BMD decreased significantly at the lumbar spine in all groups except for the combination of 1-mg estradiol and 0.5-mg norethisterone acetate. However, no significant changes were found for the total hip in any group.
The incidence of side effects related to hormone use, such as edema, headache, or irregular uterine bleeding, did not differ among groups. In particular, the incidence of irregular uterine bleeding was above 50% at one month after initiating add-back therapy, but the percentage of patients who experienced uterine bleeding decreased continuously with add-back therapy until completion of GnRH agonist treatment, and rates were comparable among groups (data not shown). In addition, the endometrial thickness by ultrasonography after treatment was not different across the four groups.
This study was conducted to compare the efficacy of different add-back regimens on hypoestrogenic problems during GnRH agonist treatment in endometriosis patients. Among the tested add-back regimens, the regimen containing estradiol valerate and norethisterone acetate was considered to be beneficial in terms of quality of life, menopausal symptoms, and BMD.
To the best of our knowledge, few studies have explored the impact of add-back therapy on quality of life in endometriosis patients during GnRH agonist treatment. Only a few studies have assessed how endometriosis-related symptoms reduce quality of life and affect socio-economic burden [6,7,8,15]: one previous study compared the effects of GnRH agonists and other hormonal treatments on quality of life, including pain, sleep disturbance, and anxiety-depression in women with pelvic pain, and showed a significant improvement in quality of life after 12 months of treatment [10]. In contrast, the current study evaluated the effects of add-back therapy on quality of life in women who underwent surgery for endometrioma and received postoperative GnRH agonist for the prevention of recurrence, regardless of pain. In the present study, social relationship and environment scores decreased in patients who received estradiol valerate alone. Since the regimen did not contain progestin and the estradiol dose was equivalent to that of other regimens containing drospirenone or norethisterone acetate, it is likely that combination with progestin affects these domains. Indeed, progestins are known to be related to psychological symptoms such as mood and lability [16,17]. Even with add-back therapy, almost all quality of life domains showed decreasing trends compared to those before GnRH agonist treatment, although statistical significance was lack. These findings suggest that no current add-back regimen can sufficiently prevent the quality of life decline that results from hypoestrogenic states induced by GnRH agonist treatment.
Although the hypoestrogenic state induced by GnRH agonist suppresses endometriosis implants and reduces endometriosis-associated pain, it can also lead to symptoms related to hypoestrogenism. In the current study, MRS scores did not increase significantly with add-back therapy during GnRH agonist treatment, and no difference was found among different regimens at each time point. Changes in the MRS scores of endometriosis patients on add-back therapy with GnRH agonist have been rarely studied. A previous study investigated health-related quality of life using MRS in patients with GnRH agonist-induced pseudomenopause [18] and reported that patients with pseudomenopause by three cycles of GnRH agonist treatment had lower MRS scores than those of surgical menopause patients.
Even with add-back therapy, BMD decreased significantly at the lumbar spine by a similar extent in all groups except the group with a combination of estradiol and norethisterone acetate. GnRH agonist treatment has been reported to induce a dramatic decrease in BMD, which can reach 4% to 5% at the lumbar spine in 6 months [19,20,21], and greater than in the early months of natural menopause. In the present study, the mean BMD change after six cycles of GnRH agonist treatment with add-back therapy was -2.85±3.14% at the lumbar spine in all patients. Consistent with our results, add-back therapies in combination with GnRH agonist treatment reduced BMD decreases in endometriosis by 1.9% after 12 months of estradiol [22] or by 1.1% after 6 months of tibolone [23] in previous studies. However, studies comparing bone loss between add-back regimens are still limited. In a small series, Leather and colleagues noted that BMD at the lumbar spine was preserved with 2 mg daily of estradiol valerate and 5 mg of cyclic norethisterone acetate in patients receiving GnRH agonist during a 6-month clinical trial [24]. Current results suggest that the use of estradiol valerate and norethisterone acetate could be more effective than other add-back regimens for bone loss prevention, if confirmed in larger trials. This difference might result from different properties of progestins, since norethindrone acetate has estrogenic and androgenic effects which can lead to more beneficial effects on bone compared to other progestins [25].
This is a unique study that directly compared the efficacy and effect on quality of life of four different add-back regimens. However, the present study has some limitations with respect to study design (no randomization) and small number of study participants. However, current number of study participants for each group is sufficient to detect any significant difference in BMD changes among groups by statistical power analysis. In addition, limited numbers of add-back regimens were included in this study, although these regimens were most commonly used in our country. Finally, not all factors which can affect BMD, such as calcium or vitamin D intake, were addressed. However, a short duration of the study and age of the study subjects suggest that changes in BMD might result from difference in regimens.
In conclusion, this study suggests that an add-back regimen containing estradiol valerate and norethisterone acetate may have better efficacy in terms of hypoestrogenism-associated problems. A large-scaled randomized controlled trial is necessary to confirm our findings in the future.
References
2. Dlugi AM, Miller JD, Knittle J. Lupron depot (leuprolide acetate for depot suspension) in the treatment of endometriosis: a randomized, placebo-controlled, double-blind study. Lupron Study Group. Fertil Steril 1990;54:419-427. PMID: 2118858.


3. Steingold KA, Cedars M, Lu JK, Randle D, Judd HL, Meldrum DR. Treatment of endometriosis with a long-acting gonadotropin-releasing hormone agonist. Obstet Gynecol 1987;69(3 Pt 1):403-411. PMID: 2950349.

4. Al Kadri H, Hassan S, Al-Fozan HM, Hajeer A. Hormone therapy for endometriosis and surgical menopause. Cochrane Database Syst Rev 2009;CD005997PMID: 19160262.


5. ACOG Committee on Practice Bulletins: Gynecology. ACOG practice bulletin. Medical management of endometriosis. Number 11, December 1999 (replaces Technical Bulletin Number 184, September 1993). Clinical management guidelines for obstetrician-gynecologists. Int J Gynaecol Obstet 2000;71:183-196. PMID: 11186465.


6. Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27:1292-1299. PMID: 22422778.


7. Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96:366-373. e8PMID: 21718982.



8. Fourquet J, Baez L, Figueroa M, Iriarte RI, Flores I. Quantification of the impact of endometriosis symptoms on health-related quality of life and work productivity. Fertil Steril 2011;96:107-112. PMID: 21621771.



9. Warnock JK, Bundren JC. Anxiety and mood disorders associated with gonadotropin-releasing hormone agonist therapy. Psychopharmacol Bull 1997;33:311-316. PMID: 9230649.

10. Bergqvist A, Theorell T. Changes in quality of life after hormonal treatment of endometriosis. Acta Obstet Gynecol Scand 2001;80:628-637. PMID: 11437721.


11. Kiilholma P, Tuimala R, Kivinen S, Korhonen M, Hagman E. Comparison of the gonadotropin-releasing hormone agonist goserelin acetate alone versus goserelin combined with estrogen-progestogen add-back therapy in the treatment of endometriosis. Fertil Steril 1995;64:903-908. PMID: 7589632.


12. Riis BJ, Christiansen C, Johansen JS, Jacobson J. Is it possible to prevent bone loss in young women treated with luteinizing hormone-releasing hormone agonists. J Clin Endocrinol Metab 1990;70:920-924. PMID: 2138631.


13. Taskin O, Yalcinoglu AI, Kucuk S, Uryan I, Buhur A, Burak F. Effectiveness of tibolone on hypoestrogenic symptoms induced by goserelin treatment in patients with endometriosis. Fertil Steril 1997;67:40-45. PMID: 8986681.


14. Heinemann LA, Potthoff P, Schneider HP. International versions of the Menopause Rating Scale (MRS). Health Qual Life Outcomes 2003;1:28PMID: 12914663.



15. Fourquet J, Gao X, Zavala D, Orengo JC, Abac S, Ruiz A, et al. Patients' report on how endometriosis affects health, work, and daily life. Fertil Steril 2010;93:2424-2428. PMID: 19926084.



16. Bethea CL, Pecins-Thompson M, Schutzer WE, Gundlah C, Lu ZN. Ovarian steroids and serotonin neural function. Mol Neurobiol 1998;18:87-123. PMID: 10065876.


17. Epperson CN, Wisner KL, Yamamoto B. Gonadal steroids in the treatment of mood disorders. Psychosom Med 1999;61:676-697. PMID: 10511016.


18. Bhattacharya SM. Health-related quality of life following surgical menopause and following gonadotrophin releasing hormone analogue-induced pseudomenopause. Gynecol Endocrinol 2009;25:621-623. PMID: 19526395.


19. Roux C, Pelissier C, Listrat V, Kolta S, Simonetta C, Guignard M, et al. Bone loss during gonadotropin releasing hormone agonist treatment and use of nasal calcitonin. Osteoporos Int 1995;5:185-190. PMID: 7655179.


20. Moghissi KS, Schlaff WD, Olive DL, Skinner MA, Yin H. Goserelin acetate (Zoladex) with or without hormone replacement therapy for the treatment of endometriosis. Fertil Steril 1998;69:1056-1062. PMID: 9627292.


21. Franke HR, van de Weijer PH, Pennings TM, van der Mooren MJ. Gonadotropin-releasing hormone agonist plus "add-back" hormone replacement therapy for treatment of endometriosis: a prospective, randomized, placebo-controlled, double-blind trial. Fertil Steril 2000;74:534-539. PMID: 10973651.


22. Fernandez H, Lucas C, Hedon B, Meyer JL, Mayenga JM, Roux C. One year comparison between two add-back therapies in patients treated with a GnRH agonist for symptomatic endometriosis: a randomized double-blind trial. Hum Reprod 2004;19:1465-1471. PMID: 15105403.


23. Lindsay PC, Shaw RW, Bennink HJ, Kicovic P. The effect of add-back treatment with tibolone (Livial) on patients treated with the gonadotropin-releasing hormone agonist triptorelin (Decapeptyl). Fertil Steril 1996;65:342-348. PMID: 8566259.


24. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with "add-back" estrogen therapy. Obstet Gynecol 1993;81:104-107. PMID: 8416441.

25. Onobrakpeya OA, Fall PM, Willard A, Chakravarthi P, Hansen A, Raisz LG. Effect of norethindrone acetate on hormone levels and markers of bone turnover in estrogen-treated postmenopausal women. Endocr Res 2001;27:473-480. PMID: 11794470.


Fig. 1
Mean percentage changes of bone mineral densities (BMDs) at the lumbar spine and total hip in different add-back regimens after 6 cycles of gonadotropin-releasing hormone agonist. No difference was found at both sites across the four groups. Only estradiol (E2)+norethisterone acetate (NETA) group did not show a significant decrease at the lumber spine. DRSP, drospirenone. *P <0.01 vs. baseline.

-
METRICS

-
- 13 Crossref
- 4,016 View
- 56 Download
- Related articles in Obstet Gynecol Sci