|
 |
- Search
| Obstet Gynecol Sci > Volume 58(6); 2015 > Article |
Abstract
High-intensity focused ultrasound (HIFU) has been regarded as a non-surgical, minimally invasive therapeutic option for patients who prioritize uterus-conservation. Although many studies have shown that HIFU therapy is a safe and effective treatment of uterine fibroid, not all fibroids are suitable for HIFU due to risks of serious complications. We experienced three cases of complications after the HIFU ablation for huge uterine fibroids, including two cases of rapid myoma enlargement and one case of heavy vaginal bleeding.
In the conservative management of uterine fibromas, high-intensity focused ultrasound (HIFU) ablation is considered to be one of the minimally invasive options for patients who prioritize uterus preservations. Although it is safe and effective, the HIFU ablation of the uterine fibroid is known to cause complications such as abdominal edema, skin burn, vaginal discharge, and abdominal pain [1]. Given the lack of reports on serious HIFU complications, many clinicians practice HIFU treatment without being properly concerned about appropriated patient selections. Furthermore, the data on the long-term impact of HIFU therapy on fertility are still insufficient, and it is also stated in the textbook that the HIFU procedure is not recommended for women wishing future fertility [2].
We present three cases of complications after HIFU ablation for huge uterine myoma in unmarried women who want future fertility, including two cases of rapid myoma enlargement and one case of heavy vaginal bleeding. Therefore, we report these cases with a brief review of the literature.
A 27-year-old unmarried woman visited Seoul St. Mary's Hospital with abdominal discomfort due to huge uterine mass and urinary frequency. She had previously received ultrasound-ultrasound (US)-guided HIFU ablation twelve times over 29 months from March 2012 to August 2014 at a different hospital.
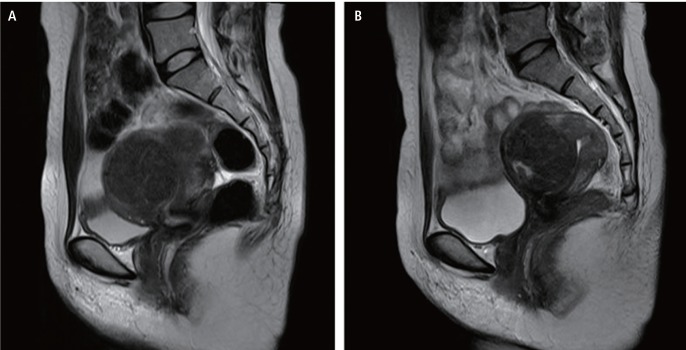
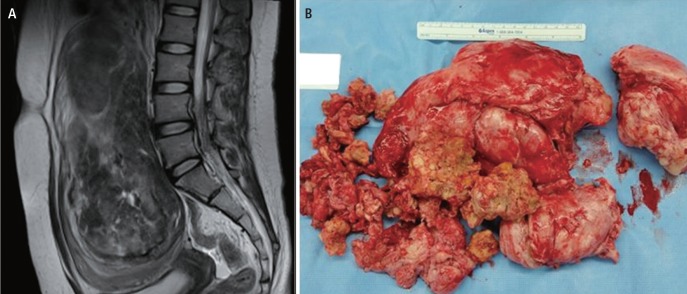
The initial magnetic resonance image (MRI) before HIFU ablation showed a 15-cm-sized well-defined hypointense mass in the posterior wall of the uterine body and fundus on T2-weighted imaging. However, the follow-up MRI after twelve HIFU ablation showed an 20-cm-sized heterogeneous myoma (Fig. 1A), extending to the renal hilum level superiorly, with a large area of nonenhancing portion in T2-weighted and post-gadolinium images, suggesting degenerative change (Fig. 1B).
The initial hemoglobin level was 10.7 g/dL and the hematocrit was 35.7%. The white blood cell count was 6.09├Ś103/mm and CA-125 level was 29.28 U/mL. The results of biochemical and coagulation test were all normal.
She underwent transabdominal myomectomy in December 2014. During the surgery, we observed that her uterus was enlarged to the size of a fetal head and the 20-cm myoma was severely necrotized. Also, the right fallopian tube was distorted and dilated due to the severely enlarged uterus. We removed the myoma and sutured the uterus with multilayer technique. We also repaired the distorted right side adnexa by suture. The final pathologic diagnosis revealed it a leiomyoma and the patient was discharged on the fourth day after the operation without any complications.
A 41-year-old unmarried woman was referred to St. Mary's Hospital with heavy vaginal bleeding and dizziness. In her history, she underwent US-guided HIFU ablation at a different hospital to remove a 5.5-cm-sized uterine submucosal myoma showing menorrhagia (Fig. 2A) in February 2014.
However, 8 months after the procedure, her unusual vaginal bleeding was not resolved and her laboratory tests showed anemia with a low hemoglobin level (5.0 mIU/L). The follow-up MRI after HIFU showed 6.1-cm-sized bulging mass with heterogeneous enhancement in the anterior wall of the uterine body on T1-weighted imaging and focal high intensity on T2-weighted imaging (Fig. 2B), signifying a probable finding of degenerative change. She received a transfusion of packed red blood cell in another hospital, and her hemoglobin level on her arrival to St. Mary's Hospital was 9.2 mIU/L.
So we planned to operate the myoma with robot-assisted laparoscopic myomectomy, and started ulipristal acetate medications until the operation day. However, while taking ulipristal acetate for 2 months, she visited the emergency department of our hospital with heavy vaginal bleeding on January 2015. Her hemoglobin level had decreased to 5.4 mIU/L again and she received transfusion of four pints of packed red blood cell.
But her vaginal bleeding was not improved after three days of hormone therapy and underwent emergency uterine artery embolization. On the next day of the procedure, the vaginal bleeding had resolved and her hemoglobin level showed much improvement to 11.2 mIU/L.
A 45-year-old, unmarried woman, gravida 0 with no sexual experience, was referred to our hospital complaining abdominal discomfort due to an oversized uterine myoma. She had a history of myomectomy 10 years ago and another intramural myoma measuring 12-cm 4 years later, for which she under
went one time of US-guided HIFU ablation at another hospital in May 2011.
Two years after the HIFU, a repeated sonogram in January 2013 showed marked interval increase in the size of the uterine myoma with extensively cystic degeneration. A pelvic MRI taken at St. Mary's Hospital showed a 16-cm-sized heterogeneous mass on the anterosuperior aspect of uterine wall (Fig. 3A) and a 6.8-cm-sized subserosal myoma along the left lateral wall of the uterine body.
The initial hemoglobin level was 13.9 g/dL and the hematocrit was 41.3%. The white blood cell count was 8.6├Ś103/mm and CA-125 level was 25.31 U/mL. The results of biochemical and coagulation test were all normal.
After discussing treatment options, the patient was scheduled for a transabdominal myomectomy. During the surgery, we observed that her uterus was enlarged to 20-week pregnancy size and adhered tightly to the bladder serosa. The largest myoma was degenerated severely. We removed ten myomas in total, including a 15-cm-sized myoma (Fig. 3B, C), with intact both adnexae. The operation was uneventful, and the patient was discharged on the fourth day after the operation. The final pathologic diagnosis was leiomyoma with degeneration, and the patient has had no complication for a year since the surgery.
Uterine fibroids are common benign tumors in premenopausal women. The patients present variable symptoms such as dysmenorrhea, menorrhagia, frequency of urination, and lower pregnancy rate, depending on the size and location of the fibroids [3]. There are many treatment options available for uterine fibroid, from surgical treatment such as hysterectomy and myomectomy, to minimally invasive or medical therapy.
As a minimal invasive treatment option, HIFU therapy is receiving more and more attention for its ability to induce coagulative necrosis at a precise focal point without harming overlying and adjacent structures. Moreover, high-intensity ultrasonic beams can ablate fibroids thermally using an extracorporeal transducer without having to introduce needles or probes into the tissue [4]. Currently, MRI or US could be used for planning and real-time monitoring of the treatment [5].
Previous studies have reported that HIFU is effective and safe in the treatment of uterine fibroids. These studies present that the success rate for HIFU treatment of uterine fibroids was more than 81% to 98.4% [678]. No mortality has been reported until now, and the majority of complications were not seriously adverse. Common complications after HIFU therapy were skin disruption, subcutaneous edema, lower abdominal pain, sciatic pain, and vaginal discharge with small amount of vaginal bleeding. However, severe adverse events were also reported such as skin burn requiring surgical repair, bowel perforation, bladder injury, and deep vein thrombosis [7]. Also one study reported that about 59.3% of patients required additional treatment (hysterectomy, myomectomy, uterine artery embolization) within five years after HIFU therapy [8].
Since there have been only a few reports about serious complications after HIFU, many clinicians were not seriously concerned of the possibility of rapid growth or unresponsiveness to HIFU treatment. It is important that clinicians recognize that not all fibroids are suitable for HIFU treatment. Prior studies reported that 16% to 35% of women with symptomatic uterine fibroid were eligible for magnetic resonance-guided HIFU treatment [9,10]. In general, patients with fibroids close to the sacral bone surface, with extensive cutaneous scars, or with bowel interposition in the US beam path should be strictly excluded. Also, the presence of concomitant severe adenomyosis, pedunculated subserosal fibroid with a pedicle less than 50% of total fibroid diameter, or multiple fibroids counting more than five in numbers or over 10 cm in size should also be considered as factors limiting treatment using magnetic resonance-guided HIFU therapy [11].
In case of US-guided HIFU treatment, no serious complications have been reported until now. Also, there are no organized criteria about indications or contraindications of US-guided HIFU, although it is thought to be similar to magnetic resonance-guided HIFU. Therefore, further studies are required to determine patient selection criteria for US-guided HIFU treatment.
We experienced three cases of complications after US-guided HIFU ablation for huge uterine fibroids, including two cases of rapid increase in size of the fibroids and one case of heavy vaginal bleeding. In the first case, the patient received US-guided HIFU ablation twelve times to remove a 15-cm-sized uterine myoma without surgery. But she might not be suitable for HIFU treatment because her myoma was too large to have therapeutic efficacy. The patient in case 2 underwent emer-gency uterine artery embolization because of unresolved vaginal bleeding after HIFU ablation. Submucosal uterine myoma is not a contraindication of HIFU treatment. However clinicians should inform the patients of the possibility of heavy vaginal bleeding after HIFU ablation. The cause of failure in case 3 was that HIFU ablation was performed on an inappropriate subtype of uterine fibroid. The patient was ineligible because her myoma was 12-cm-sized subserosal myoma with a pedicle less than 50% of total myoma diameter.
Furthermore, three patients in these cases were all nulliparous unmarried women with future plans of pregnancy. As the data on the long-term impact of HIFU therapy on fertility are still insufficient, the HIFU therapy should not replace surgical fibroid removal as the treatment of choice for women who wish to have children. Bohlmann et al. [6] make a comment that HIFU treatment for fibroids can be recommended to women with fibroid-associated subfertility who strictly reject surgical treatment or those who have very inacceptably high surgical risk.
HIFU therapy was provided as an effective therapy option for women with symptomatic uterine fibroids who desire organ-preserving treatment. However, these cases show that HIFU can make some serious complications especially in women who have desire for future fertility and therefore an appropriate patient selection is an important factor in the successful outcome of HIFU treatment.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2012R1A1A3020083).
References
1. Gorny KR, Woodrum DA, Brown DL, Henrichsen TL, Weaver AL, Amrami KK, et al. Magnetic resonance-guided focused ultrasound of uterine leiomyomas: review of a 12-month outcome of 130 clinical patients. J Vasc Interv Radiol 2011;22:857-864. PMID: 21482137.



2. Parker WH. Uterine fibroid. In: Berek JS, editors. Berek & Novak's gynecology. 15th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2012. p. 464.
3. Vollenhoven BJ, Lawrence AS, Healy DL. Uterine fibroids: a clinical review. Br J Obstet Gynaecol 1990;97:285-298. PMID: 2187522.


4. Mahmoud MZ, Alkhorayef M, Alzimami KS, Aljuhani MS, Sulieman A. High-Intensity Focused Ultrasound (HIFU) in uterine fibroid treatment: review study. Pol J Radiol 2014;79:384-390. PMID: 25371765.



5. Zhou YF. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol 2011;2:8-27. PMID: 21603311.



6. Bohlmann MK, Hoellen F, Hunold P, David M. High-Intensity focused ultrasound ablation of uterine fibroids: potential impact on fertility and pregnancy outcome. Geburtshilfe Frauenheilkd 2014;74:139-145. PMID: 24741124.



7. Zhang L, Zhang W, Orsi F, Chen W, Wang Z. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J Hyperthermia 2015;31:280-284. PMID: 25609456.


8. Quinn SD, Vedelago J, Gedroyc W, Regan L. Safety and five-year re-intervention following magnetic resonanceguided focused ultrasound (MRgFUS) for uterine fibroids. Eur J Obstet Gynecol Reprod Biol 2014;182:247-251. PMID: 25445107.


9. Behera MA, Leong M, Johnson L, Brown H. Eligibility and accessibility of magnetic resonance-guided focused ultrasound (MRgFUS) for the treatment of uterine leiomyomas. Fertil Steril 2010;94:1864-1868. PMID: 19931074.


10. O'Sullivan AK, Thompson D, Chu P, Lee DW, Stewart EA, Weinstein MC. Cost-effectiveness of magnetic resonance guided focused ultrasound for the treatment of uterine fibroids. Int J Technol Assess Health Care 2009;25:14-25. PMID: 19126247.



11. Froling V, Kroncke TJ, Schreiter NF, Scheurig-Muenkler C, Collettini F, Hamm B, et al. Technical eligibility for treatment of magnetic resonance-guided focused ultrasound surgery. Cardiovasc Intervent Radiol 2014;37:445-450. PMID: 23839005.


Fig.┬Ā1
(A) Huge uterine fibroid with degenerative change in T2-weighted image after 12 times of high-intensity focused ultrasound. (B) Degenerated uterine myoma after myomectomy.
-
METRICS

-
- 10 Crossref
- 3,553 View
- 52 Download
- Related articles in Obstet Gynecol Sci
-
Prevalence of Osteoporosis Based on Bone Density Measurement in Korean Women.1999 April;42(4)