Single-port laparoscopic debulking surgery of variant benign metastatic leiomyomatosis with simultaneous lymphatic spreading and intraperitoneal seeding
Article information
Abstract
Benign metastatic leiomyomatosis (BML) is a rare disease characterized by smooth muscle cell proliferation in extrauterine sites including the lung, abdomen, pelvis, and retroperitoneum. Depending on location, BML is classified as intravenous leiomyomatosis and diffuse peritoneal leiomyomatosis. Pathogenesis of BML can be iatrogenic after previous myomectomy or hysterectomy, hormonal, or coelomic metaplasia. Treatment options are observation, hormonal suppression, and/or surgical debulking via laparotomy or laparoscopy. Laparoscopic surgery is gaining in popularity in the gynecologic field compared to laparotomic surgery and single-port laparoscopy has the benefits of cosmesis and early tissue extraction by transumbilical morcellation. We report a 39-year-old woman with BML who underwent single-port laparoscopy debulking surgery.
Introduction
Benign metastatic leiomyomatosis (BML) is defined as a muscle tumor in association with one or more smooth muscle tumors of the uterus and without evidence of any extrauterine primary site. The group of smooth muscle tumors resembling uterine leiomyoma with unusual growth patterns and distant metastasis includes diffuse peritoneal leiomyomatosis (DPL) and, lymphatic spreading and intravenous leiomyomatosis (IVL) [1]. IVL usually invades the lumen of veins and extends into the uterine vein, ovarian vein, common iliac vein, inferior vena cava and right atrium, whereas DPL has characteristics consistent with direct peritoneal seeding [2]. Leiomyomatosis is treated by observation, hormonal suppression, and/or surgical debulking via laparotomy or laparoscopy [3]. Laparoscopic surgery decreases postoperative pain and is associated with a shorter recovery period than laparotomic surgery. Although single-port laparoscopy (SPL) with a small single transumbilical incision is technically more difficult than multi-port laparoscopy, SPL has the advantages of better cosmesis and easier tissue extraction via transumbilical morcellation than multi-port laparoscopy [4]. We report a 39-year-old woman with leiomyomatosis that had features of both multiple peritoneal seeding and lymph node metastasis who underwent SPL laparoscopic debulking surgery.
Case report
A 39-year-old woman (gravida-1, para-1) was admitted to the emergency department due to severe abdominal pain. She had a history of Cesarean section 15 years prior and total hysterectomy due to a 15-cm-diameter uterine leiomyoma 4 years prior. Physical examination revealed a distended abdomen and palpable hard mass. Abdominopelvic 3-dimensional computed tomography (CT) revealed conglomerated and enlarged para-aortic lymph nodes with multiple enlarged common iliac and external iliac lymph nodes and multiple nodules scattered in the pelvis and abdomen (Fig. 1A). Chest CT showed several small nodules in both lower lobes and lingual without lymph node enlargement in the thorax (Fig. 1). We consulted a thoracic surgeon who recommended a lung biopsy was not performed, for the patient had not repiratory symptoms and she refused it. Ultrasonography-guided biopsy of the omental mass lesion showed benign spindle cell proliferation with smooth muscle differentiation. The patient was therefore transferred to the obstetrics and gynecology department. We decided to perform minimal invasive surgery, mainly for diagnostic purpose. SPL was started under general anesthesia with the patients in the Trendelburg position. A 2.5-cm vertical intraumbilical incision was made and OCTO Port system (Dalim SurgNET Corporation, Seoul, Korea) was placed in the incision. OCTO Port has a capability to do multi-tasking by using multiple tentacles that allow three laparoscopic instruments. OCTO Port was inserted and fixed to the outer ring of the wound retractor. After insufflations of carbon dioxide, a rigid 30-degree 10-mm telescope was inserted into the abdomen. We confirmed the absence of a uterus due to the previous hysterectomy. Numerous firm, smooth nodules with diameters ranging in size from 2 to 8 cm were present on the surface of the omentum, Douglas pouch, peritoneal wall, both adnexae, small bowel and lymph nodes: paraaortic, common illiac, and external illiac lymph nodes. We performed bilateral salpingo-oophorectomy, partial omentectomy, para-aortic, common iliac, and external iliac lymph node dissection and multiple mass excisions (Fig. 2). After excision of leiomyomatosis adherent to the small bowel, primary closure of the serosal layer of the small bowel was performed by an extracorporeal approach. Histological evaluation demonstrated nodular structures with spindle cells. Signs of malignancy, including nuclear atypia or mitotic activity, were not found.

Multiple metastatic leiomyomatosis in a 39-year-old woman. Contrast enhanced 3-dimensional multi-detector computed tomography images show several well-defined masses (arrows) in abdomino-pelvic cavity (A). Multiple masses are sacttered in the Douglas pouch (B), near the left adnexae (C), peritoneum (D), omentum (E), and near both external iliac vessel (F). A chest computed tomography scan revealed multiple peripheral nodules in both lower lobe and lingula of lung (G,H).
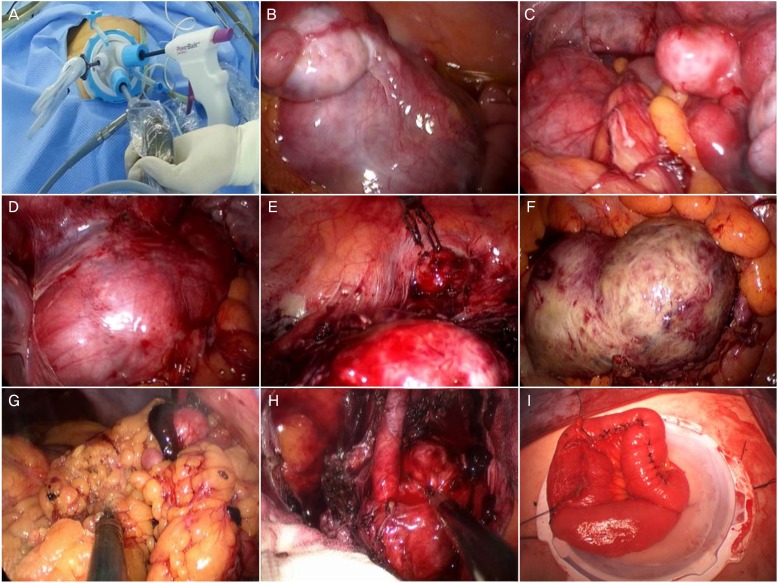
Operative findings. Single port system with OCTO Port (A). Multiple leiomyoma of near the left adnexae (B), Douglas pouch (C), near the left external iliac vessel (D), and peritoneum (E). Leiomyoma with red degeneration attached to bowel serosa and mesentery (F). Partial omentectomy (G). Para-aortic lymph node dissection (H). Extracorporeal primary repair of serosal area of small bowel after removal mass attached the small bowel (I).
The patient was given a gonadotropin-releasing hormone agonist (GnRHa) for 6 months after the surgery. There have been no subsequent signs of recurrence. There was no interval change of several small nodules in both lower lobes and lingual on the follow-up low-dose chest CT after treatment of GnRHa. There were also no subsequent signs of recurrence on the follow-up abdominopelvic CT.
Discussion
The term BML was initially coined by Steiner in 1937 and refers to multiple well-differentiated leiomyomas at sites distant from the uterus. Although this tumor type is associated with multiple distant metastases, it appears benign histologically as it is characterized by a low mitotic index, lack of nuclear pleomorphisms, and no evidence of invasion of adjacent organs [5]. Depending on tumor location, leiomyoma found outside the anatomic confines of the uterus is referred to as IVL (when found in vascular channels) and DPL (when found in the peritoneal cavity) [1]. BML metastasize to the lung most commonly, and can metastasize to other distant sites such as the heart, inferior vena cava, bone, lymph nodes, muscular tissue, and retroperitoneum infrequently [6].
There are some experimental evidences to suggest that BML and uterine leiomyoma are actually same disease. Patton et al. [7] reported that uterus and pulmonary nodule tumors had identical histology patterns (benign), immunohistochemical profiles (estrogen receptor positive, progesterone receptor positive, and very low proliferative index), and androgen receptor allelic inactivation based on molecular cytogenetic analysis. Tietze et al. [8] reported that uterine leiomyoma and pulmonary tumors showed an identical pattern of X-chromosome inactivation in comparative genomic hybridization, consistent with a monoclonal origin. These results indicate that BML is a benign disease and originates from a uterine leiomyoma.
IVL is a rare disease which is characterized by smooth muscle tumor cells invading the lumen of veins. The most common metastatic site of IVL is the lung, however it may extend to right atrium and ventricle, inferior vena cava, and tje ovarian vein. Although IVL is a benign smooth muscle tumor, extension into the cardiac cavity can cause sudden death due to mechanical obstruction [9]. Lymphatic spread of leiomyomatosis may be associated with previous surgical trauma during uterine dilatation and curettage, myomectomy, or hysterectomy. A previous study reported BML with lymphatic spread in a 27-year-old woman [10]. The authors of that study postulated that fragments of leiomyoma, detached at the time of endometrial curettage, entered the dilated lymphatic channel, and seeded several pelvic and para-aortic lymph nodes.
DPL can be differentiated from parasitic myoma, where a detached fibroid attaches to the peritoneal surface and obtains a new blood supply at a new location [11]. DPL is associated with a previous history of hysterectomy or myomectomy, high levels of exogenous and endogenous female gonadal steroids during the pregnancy, prolonged exposure to oral contraceptives or hormonal replacement therapy, or granulosa cell tumors of the ovary [11]. DPL is also associated with coexisting uterine leiomyomas and a possible association with endometriosis has been suggested by several research groups. Multipotential stem cells can differentiate not only to smooth muscle cells, but also endometrial glands and stroma [12].
BML may be misdiagnosed low-grade leiomyosarcoma; differential diagnosis of BML should therefore include metastatic leiomyomsacroma. Paley and Fornasier [13] reviewed 10 cases of leiomyosarcoma metastasizing to bone. In eight of these patients, the primary lesion of the uterus was first diagnosed as a leiomyoma, but after subsequent review, it appeared to be an underdiagnosed low-grade leiomyosarcoma. Immuno-histochemical and micro-RNA analyses revealed that the Ki67 index of BML is equivalent to that of leiomyomas and significantly less than that of leiomyosarcomas. The miRNA, miR-221, which is associated with a variety of cancers, was not detected in BML in contrast to leiomyosarcoma. Upregulation of miR-221 expression Accordingly, upregulation of may be an accurate way to differentiate leiomyosarcoma from BML; meticulous examination of specimen is needed [14].
There are no established guidelines for the management of BML. Reducing estrogen exposure is sufficient to induce regression of the disease in most cases. Treatment methods comprise extensive debulking surgical resection and medical therapies including aromatase inhibitor, selective estrogen receptor modulator, GnRHa, and/or megesterol aceate [3]. In case of resistance to endocrine therapy and/or symptomatic or intestinal involvement, extensive debulking surgical therapy is needed.
Surgical management options are laparotomy or laparoscopy. Laparoscopy is becoming preferred to laparotomy and there are a few reports of laparoscopic debulking of BML. SPL surgery has the potential to be scarless due to a relatively hidden umbilical scar, and many gynecologic surgeries for both benign and malignant diseases have been performed using SPL [15]. SPL has several advantages compared to conventional multiport laparoscopy. First, SPL had better cosmesis; second, the need for fewer trocar incisions decreases morbidity related to visceral and vascular injury during accessory trocar placement; third, tissue can be easily extracted through the umbilicus because the size of the umbilical wound is larger than that made in multiport laparoscopy [4]. However, SPL has several technical difficulties including crowding between surgical instruments during the operation, a difficult technique compared to conventional multi-port laparoscopy, and a steep learning curve. Despite these difficulties, SPL can be performed successfully with more surgical experience and newly developed instruments. We performed SPL for extracorporeal primary repair of small bowel after removal of a mass adhered to the small bowel and we extracted this mass by transumbilical morcellation.
To our knowledge, this is the first report of BML with simultaneous lymphatic spread and peritoneal seeding of leiomyomatosis. Further, this is a first report of SPL debulking surgery to treat BML. On histologic examination, leiomyomatosis near the para-aortic, common iliac, and external iliac vessel was proven to originate from the lymphatics. We failed to prove intravenous extension of leiomyomatosis because the patient refused a lung biopsy of multiple pulmonary nodules to confirm metastatic lesions.
In summary, BML is a rare disease associated with a current or prior history of uterine leiomyoma that usually follows a benign course. BML must be distinguished from low-grade leiomyosarcoma and long-term close surveillance is required for early detection of disease recurrence or distal metastases in patients with a previous history of uterine surgery.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.