Embryonic development in human oocytes fertilized by split insemination
Article information
Abstract
Objective
To compare the laboratory outcomes of intracytoplasmic sperm injection (ICSI) and conventional insemination using sibling oocytes in poor prognosis IVF cycles where ICSI is not indicated.
Methods
Couples undergoing IVF with following conditions were enrolled: history of more than 3 years of unexplained infertility, history of ≥3 failed intrauterine insemination, leukocytospermia or wide variation in semen analysis, poor oocyte quality, or ≥50% of embryos had poor quality in previous IVF cycle(s). Couples with severe male factor requiring ICSI were excluded. Oocytes were randomly assigned to the conventional insemination (conventional group) or ICSI (ICSI group). Fertilization rate (FR), total fertilization failure, and embryonic development at day 3 and day 5 were assessed.
Results
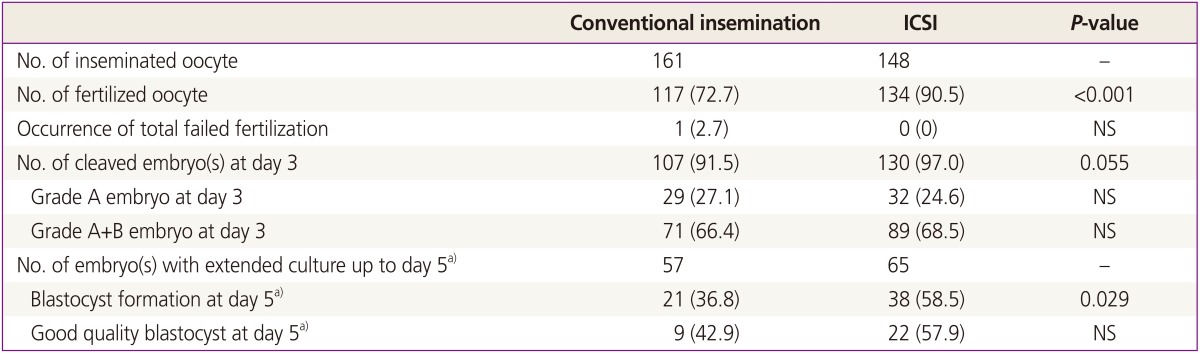
A total of 309 mature oocytes from 37 IVF cycles (32 couples) were obtained: 161 were assigned to conventional group and 148 to ICSI group. FR was significantly higher in the ICSI group compared to the conventional group (90.5% vs. 72.7%, P<0.001). Total fertilization failure occurred in only one cycle in conventional group. On day 3, the percentage of cleavage stage embryos was higher in ICSI group however the difference was marginally significant (P=0.055). In 11 cycles in which day 5 culture was attempted, the percentage of blastocyst (per cleaved embryo) was significantly higher in the ICSI group than the conventional group (55.9% vs. 25.9%, P=0.029).
Conclusion
Higher FR and more blastocyst could be achieved by ICSI in specific circumstances. Fertilization method can be tailored accordingly to improve IVF outcomes.
Introduction
Intracytoplasmic sperm injection (ICSI) was developed to overcome low fertilization rate (FR) that occurs mainly in patients with male factor infertility or prior unexplained low FR. Given the accumulating evidence of its safety and efficacy, ICSI has been widely used not only in male factor infertility but also in non-male factor infertility to prevent unexpected total fertilization failure (TFF). In 2008, ICSI was used in 69% of all in vitro fertilization (IVF) cycles in Europe, 63.2% in Australia, and 64.3% in USA [1].
In non-male factor infertility such as unexplained infertility, poor oocyte quality, low oocyte yield, or advanced maternal age, ICSI have been used to improve FR to reduce TFF [2]. However, the efficacy of ICSI in non-male factor infertility is still controversial. While several sibling oocyte studies consistently showed higher FR after ICSI compared with conventional insemination in patients with unexplained infertility [3,4,5], a randomized study showed that ICSI increases FR but no difference of pregnancy outcome in patients with non-male factor infertility [6]. Another non-randomized study found no significant difference in FR, TFF, and clinical pregnancy rate in patients with non-male factor infertility [7], and a recent randomized study also support those findings [8]. In cases of poor oocyte quality, several studies support the efficacy of ICSI [9,10]. In addition, ICSI has been investigated as a possible fertilization method in patients with lower oocyte yield [10,11,12]; however, no study supported the routine use of ICSI in patients with low oocyte yield.
Split insemination referred to dividing sibling oocytes into conventional insemination and ICSI for fertilization. Split insemination has been frequently used for selected patients with unexplained infertility; in a meta-analysis summarizing eleven retrospective sibling oocyte studies, the authors concluded that use of ICSI significantly increases FR and decreases TFF in couples with unexplained infertility [13]. However, in that meta-analysis, pregnancy outcome was not evaluated and only a few studies included reported embryonic development status including blastocyst formation and the pregnancy outcomes.
In this study, we aim to compare the laboratory outcomes of ICSI and conventional insemination using sibling oocytes (split insemination) in poor prognosis IVF cycles where ICSI is not indicated. We also report the pregnancy outcomes of split insemination in this population.
Materials and methods
IVF cycles with split insemination underwent between March 2009 and December 2013 was selected retrospectively. Split insemination was performed in couples who had at least one of the following conditions: (1) a history of more than 3 years of unexplained infertility, (2) a history of ≥3 failed intrauterine insemination, (3) leukocytospermia or wide variation in semen analysis, (4) poor oocyte quality (abnormal cytoplasm with dark colored, or granular or possessing refractile bodies) at the time of oocyte pick-up, or (5) ≥50% of embryos had poor quality (C or D grade) in previous IVF cycle(s). IVF cycles of couples with a severe male factor infertility requiring ICSI (total motile sperm count less than one million, or strict morphology less than 4%, or severe teratozoospermia [≥50%] after swim-up) were excluded. Only cycles using fresh ejaculated sperms for fertilization or insemination were included. This retrospective study was approved by the Institutional Review Board of the Seoul National University Bundang Hospital.
Ovarian stimulation was performed using recombinant follicle-stimulating hormone (Gonal-F, Serono, Geneva, Switzerland) in combination with GnRH agonist or antagonist. When at least 2 follicles reached a diameter ≥18 mm, 250 µg of recombinant human chorionic gonadotropin (Ovidrel, Serono) was injected. Transvaginal oocyte retrieval was scheduled for 35 hours after human chorionic gonadotropin triggering. Mature oocytes were randomly assigned to conventional insemination (conventional group) or ICSI (ICSI group). If oocytes were odd-numbered, one remained oocyte was assigned preferentially to conventional group.
For conventional insemination, mature oocytes were placed in the insemination medium with 50,000 or more motile sperm/mL for 1 to 4 hours after swim-up preparation. For ICSI, cumulus oocyte complexes were denuded with 80 IU/mL hyaluronidase to remove surrounding granulosa cells and were assessed nuclear maturation. Fertilization was assessed 16 to 18 hours after insemination or microinjection. Fertilization was defined as the presence of two pronuclei and the extrusion of the second polar body. FR per mature oocyte and TFF in each sibling oocytes were assessed. The TFF was defined when no oocyte formed two pronuclei in any given cycle. Blastocyst formation rate was calculated by the number of blastocysts divided by the number of cleaved embryos.
The embryos were cultured and transferred on day 3 or day 5. Cleavage stage embryos were classified on day 3 according to the alleged morphological criteria: grade A, equal-sized blastomeres and no fragments without apparent morphologic abnormalities; grade B, blastomere equal in size and <20% of fragments without apparent morphologic abnormalities; grade C, 20% to 50% fragments and irregularity of blastomeres without apparent morphologic abnormalities; grade D, >50% fragments and irregularity of blastomeres with apparent morphologic abnormalities. The blastocyst was evaluated on day 5 by the blastocyst development stage and the quality of the inner cell mass and trophectoderm [14]. A good quality blastocyst was defined as grade AA, AB, AC, BA, BB, or CA. Embryos with the best quality were transferred in each cycle.
The luteal phase was supported with a daily dose of 50 mg of progesterone in oil (Progest, Samil, Seoul, Korea) or 8% of progesterone gel (Crinone, Serono) starting on the day of the oocyte retrieval. Pregnancy was confirmed with transvaginal ultrasonography and identified the number of gestational sac(s) and the fetal heart rate(s).
All data were expressed as mean±standard deviation. IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The data were analyzed using the Wilcoxon test or the chi-square test as indicated. A P-value at <0.05 was considered statistically significant.
Results
Thirty-two patients met the inclusion criteria and underwent 37 split insemination cycles. The mean age of the women was 34.7±3.2 years (range, 27 to 42 years). The indications for IVF were; unexplained (n=21), tubo-peritoneal (n=7), polycystic ovary syndrome (n=3), endometriosis (n=3), and others (n=3). The indications for split insemination cycles were; a history of more than 3 years of unexplained infertility or of ≥3 failed intrauterine insemination (n=16); leukocytospermia or wide variation in semen analysis (n=2); poor oocyte quality (n=13); and poor embryo quality in previous cycle(s) (n=6).
The mean sperm concentration was 159±166 million/mL (range, 18 to 572 million/mL) and the mean motility was 49.3±17.0% (range, 10.5% to 80.9%). Sperm motility less than 40% was noted in eleven cycles. Mean number of mature oocyte was 8.4±5.5 per cycle; 4.4±3.6 in conventional group and 4.0±2.2 in ICSI group. A total of 309 mature oocytes were obtained and they were randomly subjected to conventional group (161 oocytes) or ICSI group (148 oocytes). The overall FR was 81.2% (251/309).
Table 1 shows the laboratory outcomes in the conventional and ICSI group. FR was significantly higher in the ICSI group (90.5% in ICSI group vs. 72.7% in conventional group, P<0.001). TFF occurred in only one case after conventional insemination. At day 3, the percentage of cleavage stage embryos was non-significantly higher in ICSI group (97.0% in ICSI group vs. 91.5% in conventional group, P=0.055). The quality of cleavage stage embryo was similar between the two groups. Blastocyst formation rate was significantly higher in the ICSI group (36.8% in conventional group and 53.5% in ICSI group, P=0.027) although the percentage of good quality blastocyst was similar.
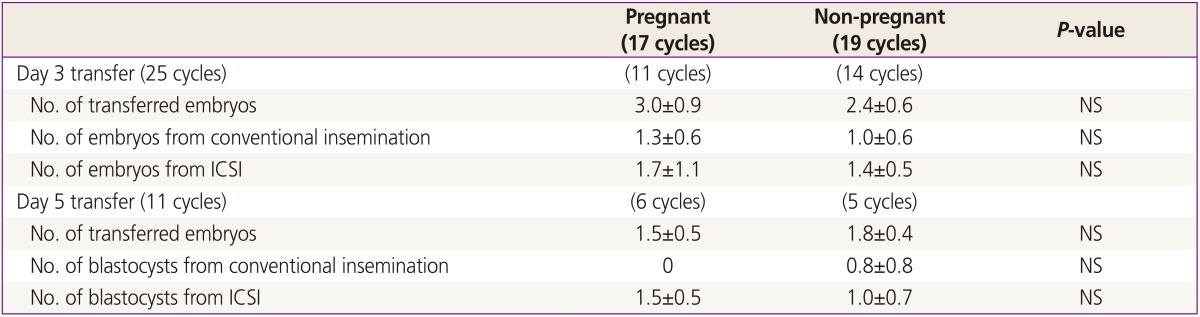
In one cycle, embryo transfer (ET) was unable owing to thin endometrium. In the 36 ET cycles, the clinical pregnancy rate was 44.0% (11/25) in day 3 transfer and 54.5% (6/11) in day 5 transfer. The implantation rate was 26.9% (18/67) in day 3 and 38.9% (7/18) in day 5 transfer. There were 3 biochemical pregnancies and 4 missed abortions before 10 weeks. When we compared the pregnant group to non-pregnant group, basal patients' characteristics including ovarian reserve, cause of infertility, semen quality and endometrial thickness were similar (data not shown). Among embryos transferred (either on day 3 or day 5), the composition of conventional and ICSI group was not different between the pregnant group and the non-pregnant group (Table 2).
Discussion
We observed that the use of ICSI resulted in significantly higher FR and blastocyst formation rate than conventional insemination in IVF cycles with a poor prognosis where ICSI is not indicated. Among the embryos transferred, the proportion of embryos fertilized with conventional IVF and ICSI was not different between the pregnant and the non-pregnant cycles.
Current indications of ICSI are solely based on the semen analysis. However, the reliability of semen analysis as a measurement of semen quality has been inconclusive because a significant number of patients with normal sperm parameters still undergo difficulty in achieving successful pregnancy [15]. Also, it is hard to interpret the results of the semen analysis when there is a wide variation among the multiple results. As described before, we excluded couples with severe male factor at enrollment, however, in 11 cycles, sperm motility was less than 40%. This might be due to the variation of their semen analysis results. Therefore, there may be additional indications of ICSI directly related to FR which current semen analysis could not find.
It was suggested that reactive oxygen species produced by seminal leukocytes (leukocytospermia) disrupt sperm quality and induce peroxidative damage of sperm membrane which would have a detrimental effect on fertilization [16]. Also, it has been reported that the thickness or color of zona pellucida and oocyte shape and quality (cytoplasmic granularity, coloration, region of organelle clustering) affect the fertilization and subsequent embryo quality and implantation rate [17,18]. However, so far, there are no routine work-ups to evaluate above abnormalities during IVF treatment. Therefore it may be possible to assume that these unrevealed abnormalities affecting fertilization may be represented as an unexplained infertility or poor prognosis such as repetitive failure of IVF and poor FR.
Some centers have used ICSI routinely for all IVF cycles to reduce TFF and transfer cancellation [1,19]. However, a definite benefit of using ICSI in non-male factor infertility is not yet rendered. Given that ICSI is a time-consuming and labor intensive technique and costs more than conventional IVF, further investigations are needed to support the routine use of ICSI in patients without male factor.
In the present study evaluating non-male factor infertility couples with poor prognosis, the FR and blastocyst formation rate was significantly higher in ICSI group which is consistent with the previous meta-analysis showing a better FR in ICSI group compared to conventional group in couples with unexplained infertility [13]. Also, the percentage of cleaved embryos at day 3 was higher in ICSI group. Therefore, our results support the application of ICSI in non-male infertility couples with poor prognosis. However, considering the small number of cycles included in this study, one should be careful to draw a definite conclusion about the use of ICSI in non-male factor infertility based on our results.
Interestingly, in the cycle where TFF occurred in conventional group, the FR in ICSI group was 100%. Although it was only one cycle with low oocytes yield (only 1 and 2 oocytes were assigned to conventional group and ICSI group, respectively), this result may also support the beneficial effect of ICSI in terms of improved fertilization in specific patient population.
Also, our finding is encouraging because the higher blastocyst formation rate may suggest a direct association between ICSI and embryo development which would lead to a better pregnancy outcome. Although we could not investigate the pregnancy rates by the group (embryos for ET were selected regardless of the group), the overall pregnancy rate was similar to the general pregnancy rate of IVF cycles in our center.
Our study has several clinical significances. First, by using sibling oocytes, we controlled the effect of oocyte and could investigate the effect of ICSI method per se. Second, while most previous studies evaluated only unexplained infertility couples, we included various clinical circumstances of poor prognosis of IVF which can expand the application of ICSI in clinical practices. Finally, this is the first to evaluate the blastocyst development rate as one of the primary outcomes which can be speculated that ICSI may have a role of propagation of embryonic development in this non-male poor prognosis population.
There are limitations in our study. Because of the small number of cycles included, various indications of ICSI and causes of infertility could not be controlled. Because the embryos transferred were selected regardless of the group, the pregnancy rate by each group could not be evaluated. Finally, the retrospective design of the study may cause underlying bias.
In conclusion, our results suggest that non-male infertility couples with poor prognosis may benefit from ICSI. ICSI procedure may enhance fertilization and stimulate embryonic development up to blastocyst stage. A prospective randomized study comparing the pregnancy rate between patients using ICSI and conventional IVF is needed to confirm the efficacy of ICSI in this population.
Acknowledgments
This work was supported by grant no. A120043 from the Korea Health Care Technology R&D Project, Ministry of Health and Welfare, Korea.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.