Survival analysis of revised 2013 FIGO staging classification of epithelial ovarian cancer and comparison with previous FIGO staging classification
Article information
Abstract
Objective
To analyze the prognostic role of revised version of International Federation of Gynecology and Obstetrics (FIGO) stage (2013) in epithelial ovarian cancer and compare with previous version staging classification
Methods
We retrospectively enrolled patients with epithelial ovarian cancer treated at Samsung Medical Center from 2002 to 2012. We reclassified the patients based on the revised FIGO staging classification.
Results
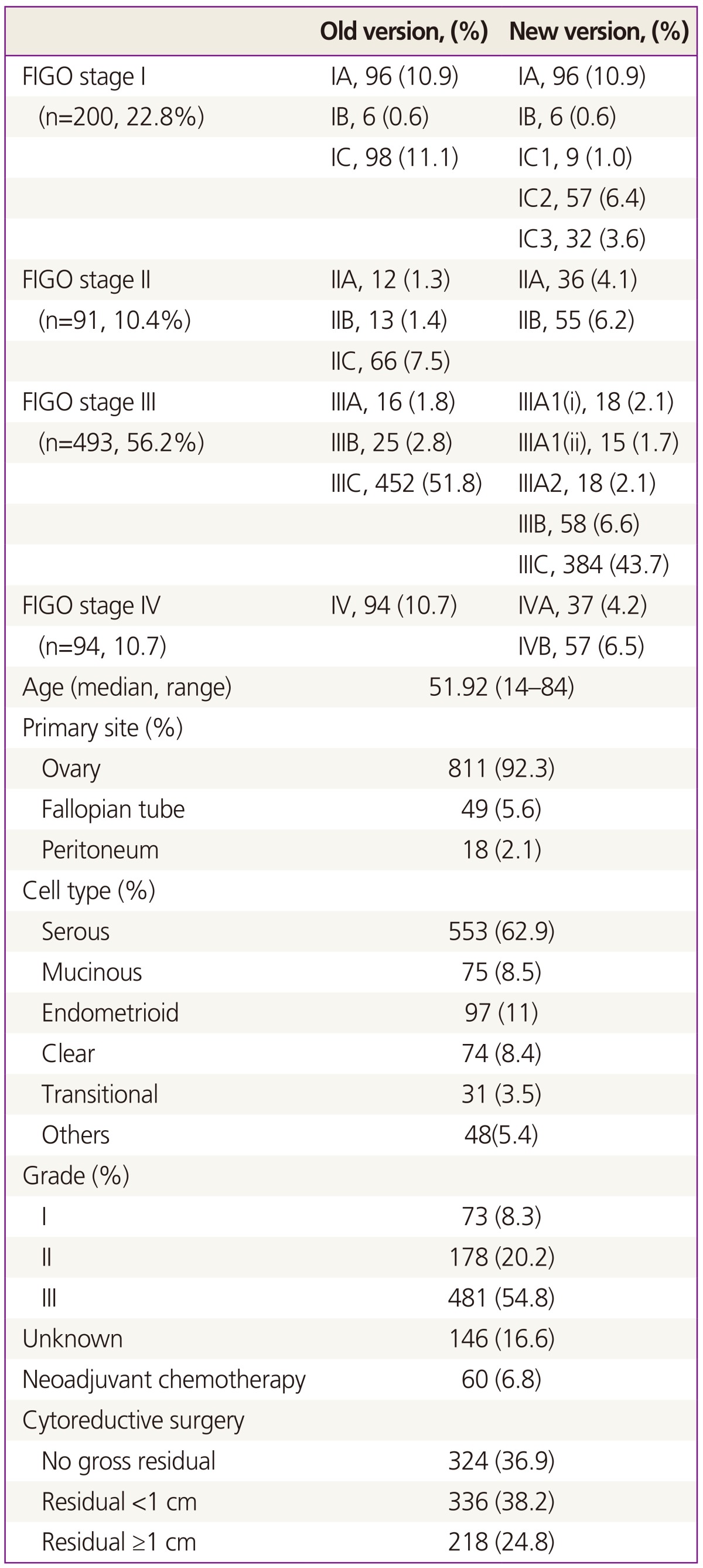
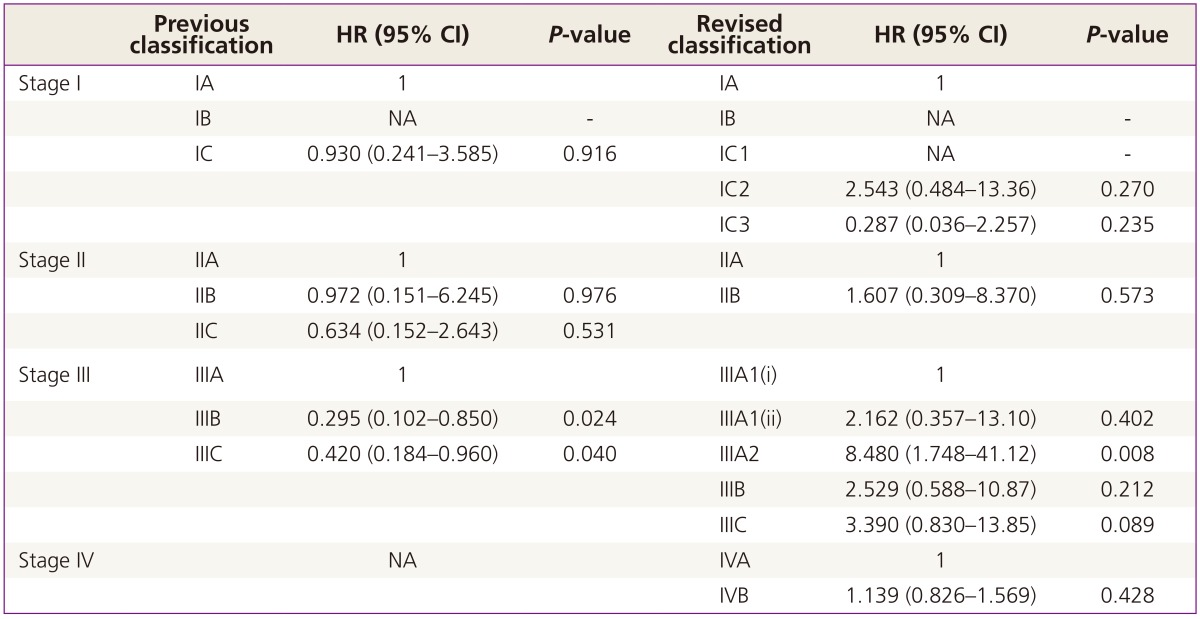
Eight hundred seventy-eight patients were enrolled (stage I, 22.8%; stage II, 10.4%; stage III, 56.2%; stage IV, 10.7%). Previous stage IC (98, 11.1%) was subdivided into IC1 (9, 1.0%), IC2 (57, 6.4%), and IC3 (32, 4.1%). In addition, previous stage IV (94, 1.7%) was categorized into IVA (37, 4.2%) and IVB (57, 6.5%) in new staging classification. Stage IIC (66, 7.5%) has been eliminated and integrated into IIA (36, 4.1%) and IIB (55, 6.2%) in revised classification. Revised FIGO stage IC3 had significant prognostic impact on PFS (hazard ratio [HR], 3.840; 95% confidence interval [CI], 1.361 to 10.83; P=0.011) and revised FIGO stage IIIC appears to be an independent, significant poor prognostic factor for PFS (HR, 2.541; 95% CI, 1.242 to 5.200; P=0.011) but not in the case of previous version of FIGO stage IIIC (HR, 1.070; 95% CI, 0.502 to 2.281; P=0.860). However, any sub-stages of both previous and revised version in stage II and IV, there was no significant prognostic role.
Conclusion
Revised FIGO stage has more progressed utility for informing prognosis than previous version, especially in stage I and III. For stage II and IV, further validation should be needed in large population based study in the future.
Introduction
Epithelial ovarian cancer (EOC) is the common female cancer worldwide and one of the most lethal malignancies [1]. The incidence is reported to be relatively high in developed countries [2] and the incidence of ovarian cancer has been increasing in Korea according to the Korea Central Cancer Registry's nationwide cancer incidence monitor since 1999 [3]. Several reasons for poor prognosis related to EOC are suspected but the advanced disease at initial visit is presumed as major factor for poor survival in EOC. Stage is known for one of the powerful prognostic factor for survival and 5-year overall survival (OS) rates of stage I and II EOC is approximately 90% and 60%, respectively, and these rates decline to about 30% in more advanced stages (III and IV) [4].
Surgical exploration and pathological staging is standard procedure for classification of EOC and International Federation of Gynecology and Obstetrics (FIGO) classification is widely used. However, there have been debates on the issues that existing FIGO staging of EOC classifies heterogeneous groups of population as same stage [5,6]. For example, FIGO stage IIIC patients with only isolated lymph node metastasis have been shown better prognosis compared to the patients with stage IIIC with peritoneal carcinomatosis or abdominal metastatic lesion more than 2 cm [6,7,8,9,10,11,12]. Unfortunately FIGO staging classification of EOC has not been revised since 1988 under these critical issues but recently, FIGO announced revised FIGO staging classification [13] reflecting recent evidences. This study is designed to investigate the clinical relevance of new FIGO staging system and to compare revised classification with previous version of FIGO staging system in EOC.
Materials and methods
1. Patients
With the institutional review board approval (2014-05-083), we retrospectively searched the patients who were diagnosed with EOC at Samsung Medical Center, Seoul, Korea from 2002 to 2012. Using the electrical medical records, data of patients such as age, stage, cell type, tumor grade, adjuvant chemotherapeutic regimen, type of surgery, surgical outcomes, etc. were gathered. The patients who had synchronous cancers were excluded. All cases were staged based on the operation records and final pathological reports according to last and revised versions of FIGO staging classification, respectively. Stage IC1 was reclassified for patients with iatrogenic rupture occurred during operation. Stage IC1 was confirmed by operation record in which incident of iatrogenic rupture was recorded separately by operator. The cases of iatrogenic rupture without previously proven positive result of malignancy for washing cytology were classified as stage IC1. The cases with positive result of malignancy for washing cytology were subdivided as stage IC3 irrelevant to status of iatrogenic rupture. Regarding stage IV, clinically suspected lung lesions on imaging studies such as computed tomography, magnetic resonance imaging, and positron emission tomography or cyto-pathological confirmation of distant organs was required for diagnosis. Primary tubal or peritoneal cancers were also included in this study. Surgical outcomes were categorized as no gross residual, optimal residual (<1 cm), and suboptimal (≥1 cm). Complete surgical staging includes washing cytology, hysterectomy, bilateral salpingo-oophporectomy, pelvic and/or para-aortic lymphadenectomy, omentectomy, and multiple biopsies on suspicious lesions. For suspected stage I EOC, fertility saving or comprehensive surgical staging was permitted via laparotomy or laparoscopy based on the attending physicians' preference. Adjuvant chemotherapy consisted of platinum based chemotherapy in all cases. Adjuvant chemotherapy can be omitted in the case of stage IA or IB with grade 1. Platinum resistance was defined as less than 6months of platinum free interval. Patients were followed up every 3 months for the first 2 years, then 6 months for up to 5 years and annually thereafter. Patients were monitored based on clinical, radiological, biochemical and imaging techniques.
2. Statistical analysis
The Kaplan-Meier method was used to estimate progression free survivals (PFSs) and OSs and comparison of survival curves between groups were carried out with log-rank test. We defined PFS as the time from the initial treatment to relapse or the last follow-up visit; OS was the time from the initial treatment to death or the last follow-up visit. Multivariate analyses of prognostic factors were carried out using Cox regression models. Factors included in multivariate analysis in stage I were age, cell type (serous vs. non-serous), grade, surgical staging methods (complete staging vs. comprehensive staging), and platinum sensitivity. For each stage II, III, and IV, multivariate analysis was performed by adjusting age, cell type (serous vs. non-serous), grade, and surgical outcomes (no gross residual, residual <1 cm, and residual ≥1 cm), and platinum sensitivity. Statistical analyses were performed by IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). A P-value of ≤0.05 was considered statistically significant and all P-values were two-sided.
Results
Overall, we were able to enroll 878 patients with EOC. The basic characteristics of patients are presented in Table 1. The median age of the population was 51.9 years old with the range of 14 and 84. Most of the patients (66.9%, 587/878) were diagnosed as advanced disease (stage III and IV) and portion of stage II was the least (10.4% ,91/878). 92.3% of patients (811/878) had primary site from ovary, 5.6% (49/878) from fallopian tube, and 2.1% (18/878) from peritoneum. Serous adenocarcinoma (62.9%, 553/878) and grade III (54.8%, 481/878) was the most common type for histology and tumor grade, respectively. For treatment, 60 patients (6.8%) had neoadjuvant chemotherapy and 52 patients (5.9%) had surgical treatment alone without chemotherapy. Optimal surgical cytoreduction was achieved in 75.1% (660/878) in all patients and 63.4% (372/587) among stage III and IV. The median follow up time was 42.1 month with the range of 0.2 to 136.6.
Compared to previous FIGO staging system, revised one is divided into more sub-stages. Previous stage IC (98, 11.1%) subdivided into IC1 (9, 1.0%), IC2 (57, 6.4%), and IC3 (32, 4.1%). In addition, previous stage IV (94, 1.7%) was categorized into IVA (37, 4.2%) and IVB (57, 6.5%) in new staging classification. Stage IIC (66, 7.5%) has been eliminated and integrated into IIA (36, 4.1%) and IIB (55, 6.2%) in revised classification. Redistribution of stage III has been observed in new staging classification. Number of patients with stage IIIC in previous staging system (452, 51.8%) decreased in new staging system (384, 43,7%). Relatively, combined number of patients with stage IIIA1(i) (18, 2.1%), IIIA1(ii) (15, 1.7%), IIIA2 (18, 2.1%), IIIB (58, 6.6%) was increased in new staging system (Table 1).
1. Stage I and II
The median age of the patients with stage I was 45.5 years old (range, 14 to 81). Among the EOC patients with stage I, clear cell (20.0%, 40/200), endometrioid (23.0%, 46/200) and mucinous (28.5%, 57/200) were relatively common histologic type compared with whole population and serous type consisted of 20.5% (41/200) in stage I. Number of low grade tumor was also higher in stage I (grade I, 27.5%, 55/200; grade II, 24.0%, 48/200) and about one third of the patients (37.5%, 75/200) had comprehensive surgical management including fertility saving surgery. There was no recurrence or death in FIGO stage IB of previous version and FIGO stage IB and IC1 of revised version. In univariate analysis, either previous or revised FIGO stages did not show statistical significance in terms of PFS and OS (Fig. 1). When we performed multivariate analysis to adjust age, cell type (serous vs. non-serous), grade, and surgical staging methods (complete staging vs. comprehensive staging), only revised FIGO stage IC3 had significant prognostic impact on PFS (hazard ratio [HR], 3.840; 95% confidence interval [CI], 1.361 to 10.83; P=0.011), but not on OS (Table 1).

Comparison of previous and revised staging classification of stage I ovarian cancer with progression free survival (PFS) and overall survival (OS). (A) Old version and (B) revised version.
The median age of the patient with stage II was 49.0 years old (range, 31 to 77). Serous histologic type was the most common (47.3%, 43/91) followed by endometrioid (19.8%, 18/91), mucinous (11.0%, 10/91), and clear cell (7.7%, 7/91). 63.7% of patients (58/91) showed grade III and only 6.6% (6/91) had grade I. Most of the patients received complete surgical staging (78.0%, 71/91) and suboptimal cytoreduction was shown in 3.3% (3/91). None of the survival analyses approved prognostic role of either FIGO stage classifications in terms of PFS and OS in stage II (Fig. 2, Tables 2 and 3).
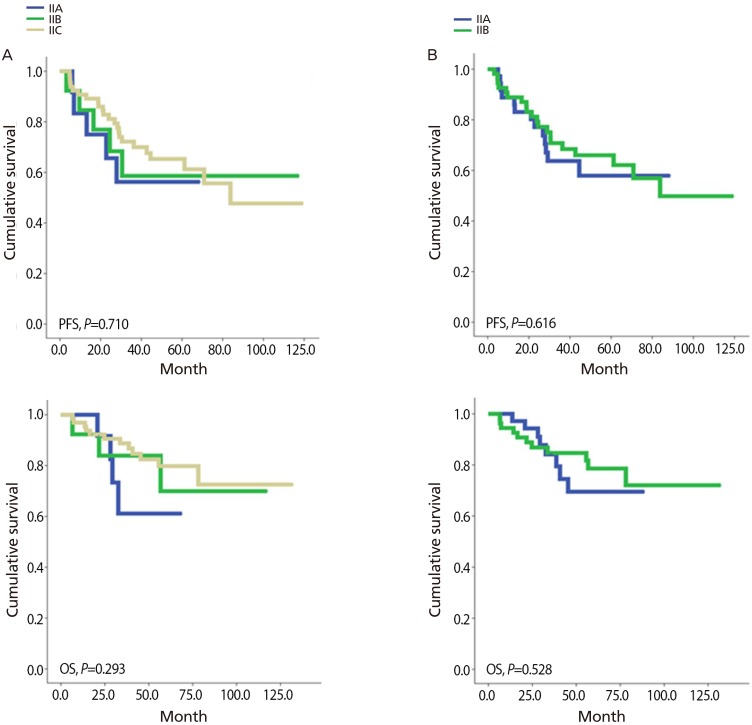
Comparison of previous and revised staging classification of stage II ovarian cancer with progression free survival (PFS) and overall survival (OS). (A) Old version and (B) revised version.
2. Stage III and IV
The median age of patients with FIGO stage III was 53 years old with the range of 22 to 84. Serous histologic type (80.9%, 399/493) and grade III (78.3%, 386/493) were most common. 5.7% (28/493) had neoadjuvant chemotherapy and suboptimal cytoreduction was shown in 35.3% (174/493). As shown in Fig. 3, revised FIGO stage classification is associated with significant stratification among stage III patients based on PFS (P=0.005) and OS (P=0.025). These findings were not observed in previous version of FIGO stage. After adjusting age, cell type (serous vs. non-serous), grade, and surgical outcomes (no gross residual, residual <1 cm, and residual ≥1 cm), revised FIGO stage IIIC appears to be an independent, significant poor prognostic factor for PFS (HR, 2.541; 95% CI, 1.242 to 5.200; P=0.011) (Table 2) but not in previous version of FIGO stage IIIC (HR, 1.070; 95% CI, 0.502 to 2.281; P=0.860) (Table 2). In terms of OS, the significant prognostic impact of revised FIGO stage IIIC, observed in PFS, turned out to be not significant, just showing trend of poor survival (HR, 3.390; 95% CI, 0.830 to 13.85; P=0.089).

Comparison of previous and revised staging classification of stage III ovarian cancer with progression free survival (PFS) and overall survival (OS). (A) Old version and (B) revised version.
The median age of patients with stage IV was 56.0 years old with the range of 23 to 81. Majority of stage IV patients had grade III (91.5%, 86/94) and serous histologic type (74.5%, 70/94) ovarian cancer. About one fourth (24.5%, 23/94) had neoadjuvant chemotherapy and optimal cytoreduction was achieved in 56.4% (53/94). When stage IV patients were divided into IVA and IVB according to revised FIGO stage, we could not find any differences in terms of proportion of neoadjuvant chemotherapy and optimal cytoreduction between two groups. As shown in Fig. 4, we could not find any statistical significant survival differences in PFS and OS and these non-significant findings still remained in multivariate analysis as well (Tables 2, 3).
Discussion
In this study, we could find that revised 2013 FIGO staging classification in EOC is acceptable and has an independent prognostic role especially in IC3 and IIIC, which were not shown in IC and IIIC of previous FIGO stage. However, the prognostic significance remains uncertain in stage II and IV.
EOC is staged surgically and surgical staging should be confirmed based on pathological findings. The major role of staging system is not only to provide universal terminology to be able to use in different centers worldwide, but also to give information about the prognosis of the patients and outcome prediction after specific treatment. Since the last version of ovarian cancer FIGO staging classification in 1988, there have been concerns that FIGO staging cannot delineate the heterogeneity of EOC patients especially in IC and IIIC. For example, whether intra-operative iatrogenic rupture of ovary in stage I EOC might have an effect on the survival outcome or not is long standing controversy [14,15,16,17,18]. Recent meta-analysis reported that iatrogenic rupture might not decrease recurrence compared to early-stage EOC without rupture in which complete surgical staging followed by platinum-based chemotherapy [19]. This suggests that stage IC with iatrogenic rupture might have better prognosis than the other stage IC. In our study, there is no case of recurrence in stage IC1, which showed even better prognosis than stage IA EOC, and these findings also support that iatrogenic ruptured IC EOC should have been allocated to different category. In the revised FIGO stage classification, stage IC3 became significant independent prognostic factor, as previous FIGO stage IC with iatrogenic rupture was re-categorized to IC1.
It has been also suggested that Stage IIIC in previous FIGO stage may be consisted of heterogeneous groups [5]. The most important issue that has been raised is the differences in prognosis between stage IIIC with intra-abdominal metastasis and IIIC with isolated lymph node metastasis without coelomic metastasis which is reported to be associated with better prognosis [6,7,8,9,10,11,12]. Allegedly, less than 10% of EOC extends beyond the pelvis with only retroperitoneal lymph node metastasis [20]. In our study, overall 3.9% of patients turned out to be the case which is relatively lower than previously reported. This lower rate might have been associated with comprehensive surgical approaches in clinically early stage and 37.5% patients with stage I EOC might not had complete lymph node dissection in our study. However, we found that survival benefits of stage IIIA1 and 2 are clear when compared to FIGO stage IIIC in revised FIGO stage. Stage IIIC was an independent prognostic factor for PFS in revised version but not in previous version of FIGO stage. These findings support that revised FIGO staging classification may be more feasible to predict prognosis of patients with EOC.
For stage II, we could not find any clinical benefits to predict prognosis with revised FIGO staging classification. The previous substage IIC (i.e., IIA or IIB but with tumor on surface, capsule ruptured, or ascites or positive peritoneal washing) was considered redundant and eliminated [13]. Clinically it is hard to distinguish stage IIA and IIB because serosa of fallopian tubes, uterus, and ovaries are continuum of pelvic peritoneum. And we often see the cases with peritoneal involvement not completely excluded due to severe adhesions even though peritoneal involvement was not proven pathologically. More on that, stage II is relatively rare. For example, a nationwide study [21] reported that less than 10% of the patients with EOC were stage II disease at initial diagnosis and in our data, we could also find that 10.4% (91/878) was stage II for 10 years which is less than 10 patients per year on average. And the event for recurrence or death was relatively rare which might be associated with low power to detect any survival difference in this group. Stage IV disease may be more heterogeneous than we expected. For example, a study demonstrated that OS for stage IV patients was significantly different between 2 groups based on supraclavicular lymph node metastasis (HR, 0.22; 95% CI, 0.08 to 0.63; P=0.005) and bone metastasis (HR, 3.49; 95% CI, 1.10 to 11.08; P=0.034). As the overall prognosis was extremely poor in stage IV, it might also be hard to see the survival differences between IVA and IVB in revised FIGO staging classification.
To our best knowledge, this is the first study to see the clinical relevance of revised FIGO staging classification in EOC in large population during relatively short period of time (10 years). Because EOC is known to have proven clinical factors associated with prognosis, including various biological behaviors based on different histology, tumor grade, and the amount of cytoreduction [16,17,22,23], we adjusted these factors to see the independent role of sub-stages through multivariate analysis. However, there are limitations in this study. First, there could be misplaced classification due to factors necessarily arise from retrospective study design. Comprehensive or fertility sparing surgery was permitted in this study, and there might be cases with microscopic metastasis on retroperitoneal lymph node that would be upstaged to stage IIIA1 instead of stage I. For the similar circumstances, stage III patients who did not have pleural tapping, because of asymptomatic pleural effusion, would have been changed into stage IVA. According to preoperative evaluation protocols for ovarian cancer of our department, pleural tapping was performed for cytologic result of malignancy in patients with pleural effusion observed in chest X-ray or computed tomography. However, possibility of improper classification of stage IVA as stage III, in patients with pleural effusion not shown in chest X-ray or computed tomography possibly due to minimal amount, cannot be excluded. Also, substage IC1 was decided by fact that whether the iatrogenic rupture had occurred during operation, indicated in operation record which was separately recorded by operator. However, possibility of not having recorded by operator leading to false classification still exist. That could be limitation of our study as retrospective design. Second, our positive results in PFS for revised FIGO staging classification were not seen in OS. Conversely, the opposite result was observed in OS for previous FIGO stage III. Stage IIIC was associated with better survival (in OS) than stage IIIA in multivariate analysis. It is unclear but we can assume that small number of patients with stage IIIA (1.8%, 16/878) and various subsequent therapies after progression, which was not considered in this study, might be associated with this result. For example, PFS can be sometimes used as a surrogate for OS but statistical modeling has suggested that the association between OS and PFS becomes weaker in diseases with longer survival post progression [24].
In conclusion, revised FIGO staging system of EOC has more progressed utility for informing prognosis than previous staging system, especially in stage I and III. For stage II and IV, further validation should be needed in large population based study in the future.
Notes
No potential conflict of interest relevant to this article was reported.