 |
 |
- Search
| Obstet Gynecol Sci > Volume 67(1); 2024 > Article |
|
Abstract
This systematic review and meta-analysis aimed to examine the effect of the antioxidant alpha-lipoic acid (ALA) on various cardiometabolic risk factors and hormonal parameters in patients with polycystic ovary syndrome (PCOS). We searched PubMed, EMBASE, SCOPUS, Cochrane Library, and Web of Science databases without language restrictions until May 2023 to find randomized controlled trials (RCTs) that assessed the impact of ALA supplementation on anthropometric, glycemic, lipid, oxidative stress, and hormonal parameters in women with PCOS. Outcomes were summarized using the standardized mean difference (SMD) and 95% confidence interval (CI) in a random-effects model. An I2 statistic of >60% established significant between-study heterogeneity. The overall certainty of the evidence for each outcome was determined using the grading of recommendations, assessment, development, and evaluations system. Seven RCTs met the inclusion criteria. The ALA group had significant reductions in fasting blood sugar (fasting blood sugar (FBS), n=7 RCTs, SMD, −0.60; 95% CI, −1.10 to −0.10; I2=63.54%, moderate certainty of evidence) and homeostatic model assessment for insulin resistance (homeostatic model assessment of insulin resistance (HOMA-IR), n=4 RCTs, SMD, −2.03; 95% CI, −3.85 to −0.20; I2=96.32%, low certainty of evidence) compared with the control group. However, significant differences were observed between the groups in body mass index, insulin, estrogen, follicle-stimulating hormone, luteinizing hormone, testosterone, low-density lipoprotein, high-density lipoprotein, triglyceride, total cholesterol, malondialdehyde, or total antioxidant capacity profiles. ALA supplementation improves FBS and HOMA-IR levels in women with PCOS. ALA consumption is an effective complementary therapy for the management of women with PCOS.
Polycystic ovary syndrome (PCOS) is a condition that affects the reproductive, metabolic, and hormonal systems. PCOS accounts for 50-70% of all infertility cases in women of childbearing age who are not ovulating [1-3] and affects approximately 10% of women of reproductive age worldwide, with annual medical expenses >$4 billion in the United States alone [4,5].
PCOS is diagnosed when two of the three criteria listed below are met: irregular menstrual cycles, signs of hyper- androgenism, and polycystic ovaries, while ruling out other causes of hyperandrogenism [6]. In addition to these clinical criteria, the metabolic profile of women with PCOS is significant in the development of this syndrome. High levels of insulin and insulin resistance (IR) have been recognized as crucial factors in PCOS development [7,8]. Insulin sensitizers such as biguanides and glitazones have been proposed as potential therapeutic options for managing hyperinsulinemia in patients [9]. Furthermore, women with PCOS have a high prevalence of several comorbidities, including obesity, ovarian dysfunction, dyslipidemia, hypertension, type 2 diabetes mellitus, and cardiovascular disease [10].
Oxidative stress is characterized by an overabundance of reactive oxygen species (ROS) and an imbalance between oxidants and antioxidants in the body [11]. Women with PCOS have higher levels of oxidative stress indicators in their blood than healthy individuals [12]. Although the exact mechanism of PCOS remains unclear, numerous studies have demonstrated a correlation between oxidative stress and its onset [13].
Supplements containing antioxidants have demonstrated positive outcomes in enhancing insulin sensitivity and addressing health risks in women diagnosed with PCOS [14,15]. Alpha-lipoic acid (ALA), a powerful antioxidant known for reducing oxidative stress and IR, is a notable supplement [16]. Evidence suggests that the controlled release of ALA can improve glucose regulation in women diagnosed with type 2 diabetes mellitus [17]. ALA exhibits robust antioxidant properties, efficiently counteracts ROS, and replenishes additional antioxidant molecules [18]. Moreover, the amalgamation of ALA and myo-inositol has demonstrated advantageous outcomes in countering oxidative stress and IR [19]. These findings suggest that antioxidant supplements such as ALA have the potential to improve insulin sensitivity and effectively manage the negative health impacts of PCOS.
Hence, a comprehensive evaluation was undertaken through a systematic review and meta-analysis of randomized controlled trials (RCTs) to consolidate the existing evidence on the effect of ALA intake, either alone or in combination with inositol, on various anthropometric, glycemic, lipid, and oxidative stress parameters in women with PCOS.
This investigation was conducted in accordance with the following recommendations outlined in the preferred reporting items for systematic reviews and meta-analyses statement, which serves as a guide for systematic reviews and meta-analyses [20], and was registered in the International prospective register of systematic reviews database (CRD42023422189).
We searched PubMed, EMBASE, SCOPUS, Cochrane Library, and Web of Science databases without language restriction. The following search terms were used: (“Alpha-Lipoic Acid,” “Lipoic Acid,” “Thioctic Acid,” “Inositol” “myo-inositol,” or “D-chiro-inositol”) AND (“Polycystic Ovary Syndrome,” “Stein-Leventhal Syndrome,” “Sclerocystic Ovarian Degeneration,” “Sclerocystic Ovaries,” “hyperandrogenism,” “Hypertrichosis” “Hirsutism,” “PCOS,” or “PCO”). Grey literature was searched using OpenGrey. The reference lists of the articles and related reviews were manually evaluated to identify additional relevant papers. The electronic databases were searched from their inception until May 15, 2023. Supplementary Material 1 shows the detailed strategy and syntax used to search each database.
Two researchers screened all titles and abstracts of the articles to remove any unrelated records. Full texts of the remaining records were obtained and evaluated to ensure they met the inclusion criteria. Data of interest were then extracted, and quality appraisals were performed. Any disagreements at each level were resolved through discussions with a third researcher. This systematic review included original papers that met the following criteria: (i) RCT studies, (ii) use of ALA supplementation alone or in combination with other treatments, (iii) studies conducted on women with PCOS, and (iv) studies with a treatment duration of at least 2 weeks. The exclusion criteria were as follows: (i) observational studies, case series, reviews, and case reports; (ii) studies without an appropriate control group; (iii) studies that lacked sufficient data on the variables of interest; and (vi) experimental, in vitro, and laboratory studies. The following information was extracted from the included RCTs: (i) the first author’s last name; (ii) the year of publication; (iii) the country of origin; (iv) the sample size; (v) the dose and duration of ALA supplementation; (vi) age of patients; and (vii) the main outcomes of the variables of interest, such as body mass index (BMI), fasting blood sugar (FBS), insulin, homeostatic model assessment of insulin resistance (HOMA-IR), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), malondialdehyde (MDA), total antioxidant capacity (TAC), estrogen, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone.
The Cochrane risk-of-bias assessment tool was used to evaluate systematically the risk of bias in the selected trials [21]. The following criteria were applied: adequacy of random sequence generation, allocation concealment, blinding, addressing dropouts (incomplete outcome data), selective outcome reporting, and other possible sources of bias. The overall certainty of evidence for the obtained effect size of each variable of interest was determined using the grading of recommendations,assessment, development, and evaluations (GRADE) system [22].
In anticipation of potentially high heterogeneity among the published articles, the significance of the net alteration between the ALA intake and control groups was evaluated using the standardized mean difference (SMD) and its 95% confidence interval (CI). Heterogeneity was assessed using the I2 statistic (significant when I2 was >60%) and Cochrane Q test (significant at P<0.1). The restricted maximum likelihood random effects model was used to calculate the pooled effect sizes of the variables of interest. The sensitivity of the effect size was assessed using the leave-one-out method. Statistical analysis was conducted using Stata/SE 17.0 for Windows (StataCorp, College Station, TX, USA).
The electronic database search yielded 708 relevant records. After eliminating duplicate articles and excluding non-eligible records, 19 articles were evaluated in full-text format. Finally, seven articles [23-28] met the inclusion criteria for quantitative synthesis, and 12 articles were excluded. Of the 12 excluded articles, nine were excluded because they were not RCTs. Two articles were excluded because they provided insufficient information about their studies. One article with irretrievable data was excluded after careful consideration. Fig. 1 shows the flow diagram of the inclusion process.
Table 1 summarizes the main characteristics of the included studies. Three studies were conducted in Italy [24,25,28], two in Iran [23,27], one in Russia [26], and one in Egypt [29]. All the included studies were conducted between 2015 and 2023. The sample sizes of the included studies ranged from 38 to 71 women with PCOS. The participants in the included RCTs were generally young adults aged 21-37 years and were overweight, with a BMI range of 21-30 kg/m2. The ALA dose ranged from 600 to 1,800 mg/day, with a median supplementation duration of 15 weeks (6-25 weeks). Five of the included studies evaluated the effects of ALA supplementation alone [23,26-29]. However, two of the included trials investigated the effects of ALA supplementation in combination with D-chiro-inositol [24] and myo-inositol [25]. We performed a subgroup analysis of all variables to determine the effect of ALA alone or in combination with inositol on the variables of interest in women with PCOS. Supplementary Fig. 1 shows the risk of bias assessment report for the included studies. Supplementary Fig. 2 shows the sensitivity analysis results of the included studies. Supplementary Fig. 3 shows the certainty of evidence for each variable based on the GRADE approach.
Two of the included studies evaluated the effect of ALA supplementation on lipid profiles in women with PCOS [24,28]. Their results indicated that ALA intake did not affect TC, TG, HDL, or LDL levels. Two additional studies were included in our systematic review that reported the effects of ALA supplementation on oxidative stress parameters in women with PCOS [23,27]. Both studies indicated that ALA intake in women with PCOS led to a significant decrease in MDA levels and a significant increase in TAC levels. However, the limited number of included studies prevented us from performing a meta-analysis to draw clear conclusions.
The overall findings of our meta-analysis of five trials showed no significant difference in BMI after ALA supplementation in women with PCOS (SMD, −0.06; 95% CI, −0.39 to 0.27; I2=43.46%, low certainty of evidence) (Fig. 2). No significant difference was observed after stratifying the studies based on ALA dosage and its combination with inositol (whether in combination or not) (Table 2).
The effect of ALA supplementation on glycemic parameters in women with PCOS indicated that ALA intake significantly decreased FBS (SMD, −0.60; 95% CI, −1.10 to 0.10; I2=63.54%, moderate certainty of evidence) and HOMA-IR (SMD, −2.03; 95% CI, −3.85 to −0.20; I2=96.32%, low certainty of evidence), but did not affect insulin levels (SMD, −1.48; 95% CI, −3.04 to 0.07; I2=97.66%, very low certainty of evidence) (Fig. 2). However, our sensitivity analysis indicated that the insulin levels significantly decreased after ALA supplementation when we excluded the Hamed et al. [29] study (SMD, −0.74; 95% CI, −1.40 to −0.08; Supplementary Fig. 2). The results of the subgroup analysis demonstrated that ALA supplementation alone significantly reduced FBS levels compared to ALA combined with inositol. HOMAIR was significantly decreased with ALA supplementation of ≤600 mg/day compared to >600 mg/day. Furthermore, insulin levels significantly decreased with combined ALA and inositol supplementation compared to ALA supplementation alone (Table 2).
The results of the meta-analysis indicated that ALA supplementation did not affect estrogen (SMD, −0.02; 95% CI, −0.29 to 0.25; I2=0%, low certainty of evidence), FSH (SMD, −2.16; 95% CI, −4.35 to 0.03; I2=97.95%, very low certainty of evidence), LH (SMD, −3.28; 95% CI, −6.70 to 0.15; I2=99.10%, very low certainty of evidence), and testosterone (SMD, 0.12; 95% CI, −0.20 to 0.44; I2=0%, low certainty of evidence) levels in women with PCOS than in the control group (Fig. 2). The subgroup analysis showed that FSH levels significantly decreased in individuals receiving ≤600 mg/day of ALA compared to those receiving >600 mg/day. Additionally, FSH levels significantly decreased when ALA was supplemented alone. Subgroup analysis also indicated that LH levels significantly decreased when ALA was supplemented for ≤12 weeks compared to >12 weeks. Additionally, LH levels decreased in the ≤600 mg/day dose of ALA compared to >600 mg/day dose. Finally, LH levels decreased significantly after ALA supplementation alone than after combined ALA and inositol supplementation (Table 2).
This systematic review and meta-analysis of RCTs indicate that ALA supplementation effectively reduces FBS levels (moderate certainty of evidence) and may effectively decrease HOMA-IR levels (low certainty of evidence) in women with PCOS. However, no substantial impact was observed on BMI or insulin, estrogen, FSH, LH, and testosterone levels in women with PCOS. However, the assessment using the GRADE framework suggests low and very low certainty of evidence for the above findings. Therefore, these results should be approached with caution. Furthermore, our systematic review showed no substantial influence of ALA intake on lipids (TC, TG, LDL, and HDL) or oxidative stress (MDA and TAC) parameters in women with PCOS. To our knowledge, this systematic review and meta-analysis is the first to examine the effect of ALA alone or in combination with inositol based on anthropometric, glycemic, lipid, oxidative stress, and hormonal parameters in women with PCOS.
Several studies have proposed that combining inositol with ALA may enhance its effects [30]. The influence of ALA on reproductive hormones seems minimal. However, its beneficial effects are probably restricted to metabolic aspects, specifically in women with PCOS and IR [30].
This systematic review and meta-analysis suggest that adding ALA to the diet does not affect the BMI of women with PCOS. Furthermore, no significant difference was observed in the BMI when inositol was combined with ALA at various dosages. These results contradict those of previous studies indicating a positive effect of ALA supplementation on BMI. Previous systematic reviews and meta-analyses demonstrated that ALA administration can significantly reduce BMI levels [31-33]. The limited number of included trials and different pathophysiologies of PCOS may have contributed to our inability to determine a significant effect of ALA on BMI.
Analysis of the collected data indicated that ALA supplementation did not significantly affect insulin levels. However, the effects of ALA on insulin levels were marginally insignificant, and ALA showed a non-significant trend toward decreasing insulin levels. Regarding the effect of ALA intake on insulin levels, our findings are consistent with those of Rahimlou et al. [17], who could not detect any significant effects of ALA supplementation on insulin levels. However, our results are inconsistent with those of Mahmoudi-Nezhad et al. [34], who reported a significant decrease in insulin levels following ALA administration. This inconsistency may be due to high heterogeneity among the included trials and the limited number of trials in our systematic review. Subgroup analysis of insulin indicated that combined ALA and inositol significantly decreased insulin levels compared to ALA alone. However, it is imperative to note that owing to the limited number of included studies in each subgroup, these results must be interpreted carefully, and larger studies are needed to further clarify and validate these findings.
The findings of the current systematic review on the effect of ALA on FBS are consistent with those of previous systematic reviews, demonstrating that ALA intake can lower FBS levels [17,35]. Regarding HOMA-IR, our results indicate a significant reduction after ALA supplementation in HOMAIR compared to the control group, consistent with previous studies [34].
Several explanations have been proposed for the potentially favorable effects of ALA supplementation on high FBS levels and IR [36]. Research has indicated that ALA may affect FBS levels by promoting glucose absorption through improved transportation of the glucose transporter type 4 to the membranes of fat and muscle cells [37,38]. According to Jacob et al. [39], intravenous administration of 1,000 mg ALA improved insulin sensitivity and the rate at which the body cleared metabolized insulin in patients with type 2 diabetes mellitus. Additionally, the significant effect of ALA on FBS is because of increased glucose transport activity in skeletal muscles [40,41]. However, it is crucial to acknowledge that the influence of ALA extends beyond skeletal muscles, as it inhibits gluconeogenesis and glucose production in the liver [42,43]. Furthermore, ALA exerts a partially positive impact on insulin metabolic pathways [44]. Numerous studies have indicated that ALA boosts the efficiency of distinct proteins involved in the insulin signaling pathway, such as insulin receptor, phosphatidylinositide 3-kinase [45], insulin receptor substrate 1 [46,47], and protein kinase B [48]. Owing to these effects, ALA is often described as a substance that emulates the functions of insulin. Lee et al. [49] demonstrated that ALA stimulates AMP-activated protein kinase (AMPK) activation in skeletal muscles. These results suggest that the improvement in insulin sensitivity induced by ALA was mediated by AMPK activation [50].
This systematic review and meta-analysis results indicate that ALA supplementation does not significantly affect hormonal parameters, including estrogen, FSH, LH, and testosterone levels in women with PCOS. However, subgroup analysis suggested that ALA supplementation at a dosage of <600 mg/day significantly decreased FSH and LH levels compared to a dosage of >600 mg/day. Subgroup analysis demonstrated that ALA alone significantly decreased FSH and LH levels compared to ALA combined with inositol. The subgroup analysis indicated that compared to >12 weeks, ALA supplementation decreased LH levels in ≤12 weeks. However, it is important to note that because of the limited number of included studies in each subgroup, these results must be interpreted carefully, and larger studies are needed to further clarify and validate these findings.
Our study had several limitations. First, the included RCTs originated from four countries, which may have affected the generalizability of the results. Second, although ALA demonstrated the most notable variation in supplementation, other minor modifications could complicate the effect of ALA on the cardiometabolic and hormonal aspects of individuals with PCOS. Other potential factors influencing cardiometabolic markers also exist, such as vitamin D levels and alterations in gut microbiomes. Third, certain trials lacked sufficient information regarding the characteristics of the participants, including BMI and age, making it difficult to accurately categorize these studies into specific subgroups. Fourth, some variables were available in a few studies, which impedes the ability to draw definitive conclusions and conduct more detailed subgroup analyses. Their interpretation should be exercised with extreme caution. Finally, significant variability was observed among the studies, which should be considered when interpreting the conclusions and outcomes of this meta-analysis.
ALA will beneficially reduce FBS levels and may favorably improve IR (by decreasing HOMA-IR) in women with PCOS. However, further research is necessary to validate these findings, particularly when combined with inositol and hormone supplementation. Furthermore, studies focusing on the influence of ALA on other PCOS features, such as fertility and pregnancy rates, are needed. Nevertheless, regarding clinical application, ALA is an effective complementary therapy for managing PCOS. The favorable antioxidant and anti-inflammatory properties of ALA can help decrease cardiometabolic risk factors, including IR, in women with PCOS.
Acknowledgements
The authors thank Saad Mohammed S Alqarni, Eman Abbas Zaher, and Norah Saud Arafat for support on this research.
Fig. 1
The preferred reporting items for systematic reviews and meta-analyses flow diagram. RCT, randomized controlled trial. *The electronic databases were searched from their inception until May 15, 2023.

Fig. 2
The effect of alpha-lipoic acid intake on body mass index (A), fasting blood sugar (B), insulin (C), homeostatic model assessment for insulin resistance (D), estrogen (E), follicle-stimulating hormone (F), luteinizing hormone (G), and testosterone (H) in women with polycystic ovary syndrome. SD, standard deviation; SMD, standardized mean difference; CI, confidence interval; REML, restricted maximum likelihood.
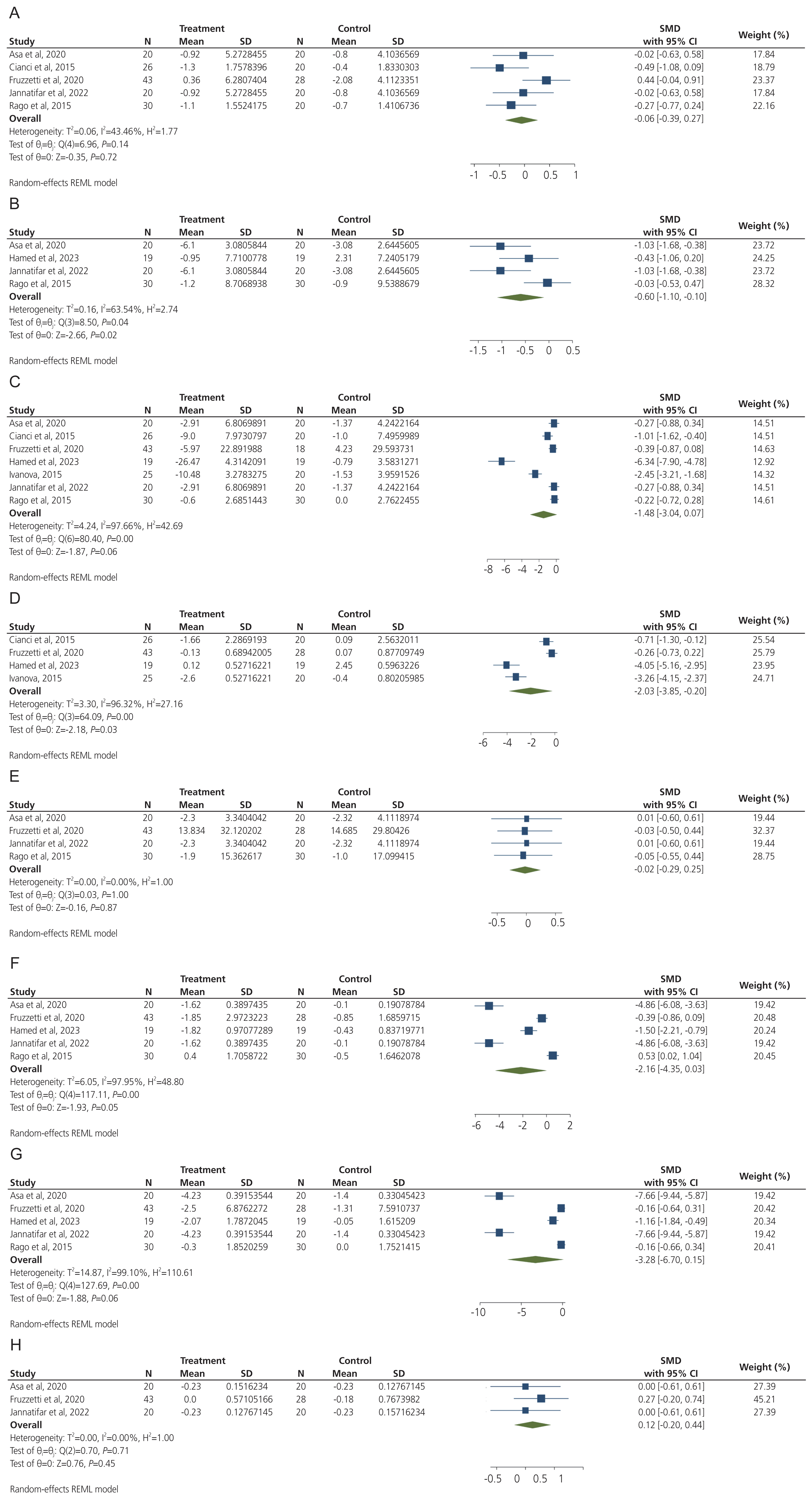
Table 1
Characteristics of included studies
| Study | Country | N | ALA dosage (mg/day) | Combination | Duration (weeks) | Age (yr) | BMI (kg/m2) | Main outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| ALA | Control | ALA | Control | |||||||
| Asa et al. [23] (2020) | Iran | 40 | 600 | None | 8 | Not reported | Not reported | 28.02±5.37 | 27.6±4.2 | ↓FBS, ↓insulin, ↓MDA, ↑TAC, ↑LH, ↔FSH, ↔E2, ↔testosterone |
|
|
||||||||||
| Cianci et al. [24] (2015) | Italy | 46 | 600 | D-chiro-inositol | 25 | 23.8±2.5 | 23.8±2.5 | 28.7±2.0 | 28.6±2.0 | ↓HOMA-IR, ↓insulin, ↓BMI, ↔TC, ↔TG, ↔HDL |
|
|
||||||||||
| Fruzzetti et al. [25] (2020) | Italy | 71 | 800 | Myo-inositol | 24 | 21.56±4.77 | 23.1±5.4 | 26.97±5.15 | 27.17±3.93 | ↔BMI, ↓FSH, ↓LH, ↓E2, ↔testosterone, ↔insulin, ↔HOMA-IR |
|
|
||||||||||
| Hamed et al. [29] (2023) | Egypt | 38 | 600 | None | 24 | 28.68±2.1 | 29.78±2.57 | 26.08±1.31 | 26.41±2.82 | ↓FBS, ↓insulin, ↔HOMA-IR, ↔FSH, ↔LH |
|
|
||||||||||
| Ivanova [26] (2015) | Russia | 45 | 600 | None | 12 | 23.9±1.9 | 25.4±2.2 | 30.7±0.6 | 30.7±0.6 | ↓insulin, ↓HOMA-IR |
|
|
||||||||||
| Jannatifar et al. [27] (2022) | Iran | 40 | 1,800 | None | 6 | 28.75±3.39 | 29.54±3.45 | 28.02±5.37 | 27.60±4.20 | ↓MDA, ↑TAC, ↓FBS, ↓insulin, ↓FSH, ↓LH |
|
|
||||||||||
| Rago et al. [28] (2015) | Italy | 65 | 800 | None | 12 | 37.1±2.7 | 36.3±2.8 | 22.3±1.5 | 21.9±1.5 | ↓insulin, ↓BMI, ↔TC, ↔TG, ↔HDL,↔ LDL, ↔FBS, ↔FSH, ↔LH,↔ E2 |
ALA, alpha-lipoic acid; BMI, body mass index; ↓, decrease; FBS, fasting blood sugar; MDA, malondialdehyde; ↑, increase; TAC, total antioxidant capacity; LH, luteinizing hormone; ↔, no change; FSH, follicle-stimulating hormone; E2, estrogen; HOMA-IR, homeostatic model assessment for insulin resistance; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 2
Subgroup analysis of all outcomes based on ALA dosage, duration of supplementation, and combination with inositol
| Variable | Number of studies | Effect size SMD | 95% CI | I2 (%) | P-value for heterogeneity |
|---|---|---|---|---|---|
| BMI | |||||
| ALA dosage | |||||
| ≤600 mg/day | 3 | −0.19 | −0.54 to 0.16 | 0 | 0.450 |
| >600 mg/day | 2 | 0.09 | −0.60 to 0.78 | 74.74 | 0.050 |
| Combination with inositol | |||||
| Yes | 2 | −0.01 | −0.92 to 0.90 | 82.92 | 0.020 |
| No | 3 | −0.13 | −0.45 to 0.20 | 0 | 0.770 |
| FBS | |||||
| Combination with inositol | |||||
| Yes | 1 | −0.39 | −0.86 to 0.09 | - | - |
| No | 3 | −2.62 | −5.24 to −0.01a) | 97.5 | <0.001 |
| HOMA-IR | |||||
| ALA dosage | |||||
| ≤600 mg/day | 3 | −2.64 | −4.63 to −0.64a) | 94.08 | <0.001 |
| >600 mg/day | 1 | −0.26 | −0.73 to 0.22 | - | - |
| Combination with inositol | |||||
| Yes | 2 | −0.45 | −0.89 to −0.01a) | 28.33 | 0.240 |
| No | 2 | −3.59 | −4.35 to −2.82a) | 16.83 | 0.270 |
| Insulin | |||||
| Duration | |||||
| ≤12 weeks | 4 | −0.78 | −1.82 to 0.27 | 91.43 | <0.001 |
| >12 weeks | 3 | −2.52 | −6.15 to 1.12 | 98.71 | <0.001 |
| ALA dosage | |||||
| ≤600 mg/day | 5 | −1.99 | −4.13 to 0.15 | 97.72 | <0.001 |
| >600 mg/day | 2 | −0.31 | −0.65 to 0.04 | 0 | 0.620 |
| Combination with inositol | |||||
| Yes | 2 | −0.67 | −1.28 to −0.07a) | 59.54 | 0.120 |
| No | 5 | −1.84 | −4.07 to 0.40 | 98.11 | <0.001 |
| E2 | |||||
| ALA dosage | |||||
| ≤600 mg/day | 2 | 0.01 | −0.42 to 0.43 | 0 | 0.1 |
| >600 mg/day | 2 | −0.04 | −0.38 to 0.30 | 0 | 0.940 |
| Combination with inositol | |||||
| Yes | 1 | −0.03 | −0.50 to 0.44 | - | - |
| No | 3 | −0.02 | −0.35 to 0.31 | 0 | 0.980 |
| FSH | |||||
| ALA dosage | |||||
| ≤600 mg/day | 3 | −3.69 | −5.93 to −1.45a) | 92.83 | <0.001 |
| >600 mg/day | 2 | 0.07 | −0.83 to 0.97 | 85.05 | 0.001 |
| Combination with inositol | |||||
| Yes | 1 | −0.39 | −0.86 to 0.09 | - | - |
| No | 4 | −2.62 | −5.24 to −0.01a) | 97.5 | <0.001 |
| LH | |||||
| Duration | |||||
| ≤12 weeks | 3 | −5.09 | −10.05 to −0.13a) | 97.58 | <0.001 |
| >12 weeks | 2 | −0.63 | −1.61 to 0.34 | 82.23 | 0.020 |
| ALA dosage | |||||
| ≤600 mg/day | 3 | −5.42 | −9.73 to −1.11a) | 96.55 | <0.001 |
| >600 mg/day | 2 | −0.16 | −0.51 to 0.18 | 0 | 0.1 |
| Combination with inositol | |||||
| Yes | 1 | −0.16 | −0.64 to 0.31 | - | - |
| No | 4 | −4.08 | −8.06 to −0.10a) | 98.71 | <0.001 |
References
1. Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol 2022;10:668-80.


2. Heshmati J, Sepidarkish M, Morvaridzadeh M, Farsi F, Tripathi N, Razavi M, et al. The effect of cinnamon supplementation on glycemic control in women with polycystic ovary syndrome: a systematic review and meta-analysis. J Food Biochem 2021;45:e13543.



3. Moradi N, Bidgoli SA, Chaichian S. Ovarian cysts disappear after 14-day oral regimen of Korean red ginseng extract in letrozole-induced polycystic ovarian syndrome. Obstet Gynecol Sci 2021;64:274-83.




4. Naz MSG, Tehrani FR, Majd HA, Ahmadi F, Ozgoli G, Fakari FR, et al. The prevalence of polycystic ovary syndrome in adolescents: a systematic review and meta-analysis. Int J Reprod Biomed 2019;17:533-42.


5. Riestenberg C, Jagasia A, Markovic D, Buyalos RP, Azziz R. Health care-related economic burden of polycystic ovary syndrome in the United States: pregnancy-related and long-term health consequences. J Clin Endocrinol Metab 2022;107:575-85.



6. Louwers YV, Laven JSE. Characteristics of polycystic ovary syndrome throughout life. Ther Adv Reprod Health 2020;14:2633494120911038.




7. Salehpour S, Hosseini S, Nazari L, Hosseini M, Saharkhiz N. The effect of vitamin D supplementation on insulin resistance among women with polycystic ovary syndrome. JBRA Assist Reprod 2019;23:235-8.



8. Karimi E, Heshmati J, Shirzad N, Vesali S, Hosseinzadeh-Attar MJ, Moini A, et al. The effect of synbiotics supplementation on anthropometric indicators and lipid profiles in women with polycystic ovary syndrome: a randomized controlled trial. Lipids Health Dis 2020;19:60.




9. Romualdi D, Versace V, Lanzone A. What is new in the landscape of insulin-sensitizing agents for polycystic ovary syndrome treatment. Ther Adv Reprod Health 2020;14:2633494120908709.




10. Glintborg D, Andersen M. Medical treatment and comorbidity in polycystic ovary syndrome: an updated review. Curr Opin Endocr Metab Res 2020;12:33-40.

11. Costantini D. Understanding diversity in oxidative status and oxidative stress: the opportunities and challenges ahead. J Exp Biol 2019;222(Pt 13):jeb194688.



12. Shan H, Luo R, Guo X, Li R, Ye Z, Peng T, et al. Abnormal endometrial receptivity and oxidative stress in polycystic ovary syndrome. Front Pharmacol 2022;13:904942.



13. Abudawood M, Tabassum H, Alanazi AH, Almusallam F, Aljaser F, Ali MN, et al. Antioxidant status in relation to heavy metals induced oxidative stress in patients with polycystic ovarian syndrome (PCOS). Sci Rep 2021;11:22935.



14. Sandhu JK, Waqar A, Jain A, Joseph C, Srivastava K, Ochuba O, et al. Oxidative stress in polycystic ovarian syndrome and the effect of antioxidant N-acetylcysteine on ovulation and pregnancy rate. Cureus 2021;13:e17887.



15. Pakniat H, Lalooha F, Movahed F, Boostan A, Khezri MB, Hedberg C, et al. The effect of ginger and metoclopramide in the prevention of nausea and vomiting during and after surgery in cesarean section under spinal anesthesia. Obstet Gynecol Sci 2020;63:173-80.




16. Zonooz SR, Hasani M, Morvaridzadeh M, Pizarro AB, Heydari H, Yosaee S, et al. Effect of alpha-lipoic acid on oxidative stress parameters: a systematic review and meta-analysis. J Funct Foods 2021;87:104774.

17. Rahimlou M, Asadi M, Banaei Jahromi N, Mansoori A. Alpha-lipoic acid (ALA) supplementation effect on glycemic and inflammatory biomarkers: a systematic review and meta-analysis. Clin Nutr ESPEN 2019;32:16-28.


18. Fogacci F, Rizzo M, Krogager C, Kennedy C, Georges CMG, Knežević T, et al. Safety evaluation of α-lipoic acid supplementation: a systematic review and meta-analysis of randomized placebo-controlled clinical studies. Antioxidants (Basel) 2020;9:1011.



19. Maciejczyk M, Żebrowska E, Nesterowicz M, Żendzian-Piotrowska M, Zalewska A. α-Lipoic acid strengthens the antioxidant barrier and reduces oxidative, nitrosative, and glycative damage, as well as inhibits inflammation and apoptosis in the hypothalamus but not in the cerebral cortex of insulin-resistant rats. Oxid Med Cell Longev 2022;2022:7450514.




20. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1.




21. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons; 2019.
22. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6.



23. Asa E, Janatifar R, Soltani MA, Verdi A, Janbazi M. Effect of alpha-lipoic acid on blood hormonal parameters and follicular fluid oxidative stress factors in infertile women with polycystic ovary syndrome undergoing intracytoplasmic sperm injection. Qom Univ Med Sci J 2020;14:49-58.


24. Cianci A, Panella M, Fichera M, Falduzzi C, Bartolo M, Caruso S. d-chiro-Inositol and alpha lipoic acid treatment of metabolic and menses disorders in women with PCOS. Gynecol Endocrinol 2015;31:483-6.

25. Fruzzetti F, Benelli E, Fidecicchi T, Tonacchera M. Clinical and metabolic effects of alpha-lipoic acid associated with two different doses of myo-inositol in women with polycystic ovary syndrome. Int J Endocrinol 2020;2020:2901393.




26. Ivanova LA. Influence of thioctic acid on the hyperinsulinemia and ovarian volume in female patients with polycystic ovary syndrome. Open J Endocr Metab Dis 2015;5:37.


27. Jannatifar R, Piroozmanesh H, Sahraei SS, Asa E. Combination of alpha lipoic acid and metformin supplement improve assisted reproductive technologies outcomes in polycystic ovary syndrome patients. Anat Cell Biol 2022;55:239-46.



28. Rago R, Marcucci I, Leto G, Caponecchia L, Salacone P, Bonanni P, et al. Effect of myo-inositol and alpha-lipoic acid on oocyte quality in polycystic ovary syndrome non-obese women undergoing in vitro fertilization: a pilot study. J Biol Regul Homeost Agents 2015;29:913-23.

29. Hamed BM, El-Sayed YAE, El-Ghazawy SS, Mohamed AI. Effects of thioctic acid on the hyperinsulinemia and ovarian volume in female patients with polycystic ovary syndrome. Egyptian J Hosp Med 2023;90:2728-33.

30. Laganà AS, Monti N, Fedeli V, Gullo G, Bizzarri M. Does alpha-lipoic acid improve effects on polycystic ovary syndrome? Eur Rev Med Pharmacol Sci 2022;26:1241-7.

31. Namazi N, Larijani B, Azadbakht L. Alpha-lipoic acid supplement in obesity treatment: a systematic review and meta-analysis of clinical trials. Clin Nutr 2018;37:419-28.


32. Kucukgoncu S, Zhou E, Lucas KB, Tek C. Alpha-lipoic acid (ALA) as a supplementation for weight loss: results from a meta-analysis of randomized controlled trials. Obes Rev 2017;18:594-601.




33. Vajdi M, Abbasalizad Farhangi M. Alpha-lipoic acid supplementation significantly reduces the risk of obesity in an updated systematic review and dose response meta-analysis of randomised placebo-controlled clinical trials. Int J Clin Pract 2020;74:e13493.



34. Mahmoudi-Nezhad M, Vajdi M, Farhangi MA. An updated systematic review and dose-response meta-analysis of the effects of α-lipoic acid supplementation on glycemic markers in adults. Nutrition 2021;82:111041.


35. Mohammadi V, Khorvash F, Feizi A, Askari G. Does alpha-lipoic acid supplementation modulate cardiovascular risk factors in patients with stroke? A randomized, double-blind clinical trial. Int J Prev Med 2018;9:34.



36. Najafi N, Mehri S, Ghasemzadeh Rahbardar M, Hosseinzadeh H. Effects of alpha lipoic acid on metabolic syndrome: a comprehensive review. Phytother Res 2022;36:2300-23.



37. Aiassa V, Del Rosario Ferreira M, Villafañe N, Eugenia D’Alessandro M. α-Linolenic acid rich-chia seed modulates visceral adipose tissue collagen deposition, lipolytic enzymes expression, insulin signaling and GLUT-4 levels in a diet-induced adiposity rodent model. Food Res Int 2022;156:111164.


38. Kaviyani N, Keshavarz SA, Abbasi B. Effect of alphalipoic acid supplementation on glycemic control in the patients with metabolic syndrome: a randomized clinical trial [Internet]. Research Square c2020 [cited 2023 May 17]. Available from: https://doi.org/10.21203/rs.3.rs-37045/v1
.

39. Jacob S, Henriksen EJ, Schiemann AL, Simon I, Clancy DE, Tritschler HJ, et al. Enhancement of glucose disposal in patients with type 2 diabetes by alpha-lipoic acid. Arzneimittelforschung 1995;45:872-4.

40. Diane A, Mahmoud N, Bensmail I, Khattab N, Abunada HA, Dehbi M. Alpha lipoic acid attenuates ER stress and improves glucose uptake through DNAJB3 cochaperone. Sci Rep 2020;10:20482.




41. Capece U, Moffa S, Improta I, Di Giuseppe G, Nista EC, Cefalo CMA, et al. Alpha-lipoic acid and glucose metabolism: a comprehensive update on biochemical and therapeutic features. Nutrients 2023;15:18.



42. Elbadawy AM, Abd Elmoniem RO, Elsayed AM. Alpha lipoic acid and diabetes mellitus: potential effects on peripheral neuropathy and different metabolic parameters. Alexandria J Med 2021;57:113-20.

43. Dugbartey GJ, Alornyo KK, Adams I, Atule S, Obeng-Kyeremeh R, Amoah D, et al. Targeting hepatic sulfane sulfur/hydrogen sulfide signaling pathway with α-lipoic acid to prevent diabetes-induced liver injury via upregulating hepatic CSE/3-MST expression. Diabetol Metab Syndr 2022;14:148.




44. Zeng M, Xu J, Zhang Z, Zou X, Wang X, Cao K, et al. Htd2 deficiency-associated suppression of α-lipoic acid production provokes mitochondrial dysfunction and insulin resistance in adipocytes. Redox Biol 2021;41:101948.



45. Lee SJ, Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG, et al. Alpha-lipoic acid inhibits endoplasmic reticulum stress-induced cell death through PI3K/Akt signaling pathway in FRTL5 thyroid cells. Horm Metab Res 2011;43:445-51.


46. Kra G, Daddam JR, Moallem U, Kamer H, Mualem B, Levin Y, et al. Alpha-linolenic acid modulates systemic and adipose tissue-specific insulin sensitivity, inflammation, and the endocannabinoid system in dairy cows. Sci Rep 2023;13:5280.




47. Mohammed MA, Mahmoud MO, Awaad AS, Gamal GM, Abdelfatah D. Alpha lipoic acid protects against dexamethasone-induced metabolic abnormalities via APPL1 and PGC-1 α up regulation. Steroids 2019;144:1-7.


48. Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 2004;10:727-33.



- TOOLS
-
METRICS

-
- 2 Crossref
- Scopus
- 1,592 View
- 146 Download
- Related articles in Obstet Gynecol Sci




















