|
 |
- Search
| Obstet Gynecol Sci > Volume 65(4); 2022 > Article |
|
Abstract
Objective
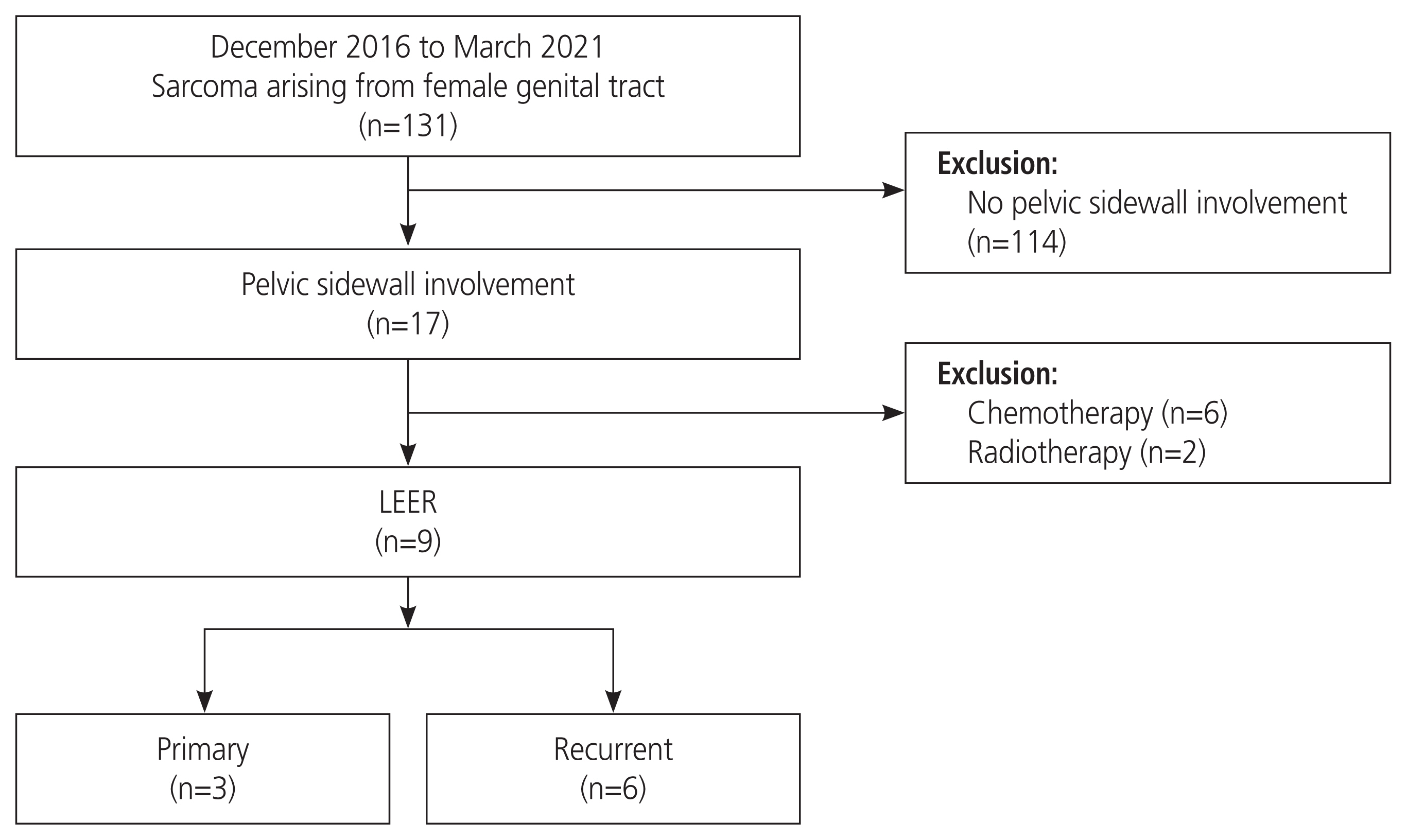
Methods
Results
Acknowledgments
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Jae-Weon Kim has been an Editorial Board of Obstetrics & Gynecology Science; however, he was not involved in the peer reviewer selection, evaluation, or decision process of this article. Otherwise, no other potential conflicts of interest relevant to this article were reported.
Table┬Ā1
| Characteristic | Value (n=9) |
|---|---|
| Age (yr) | 56 (22-65) |
| Comorbidity | |
| ŌĆāYesa) | 5 (55.5) |
| ŌĆāNone | 4 (44.4) |
| Histology | |
| ŌĆāCarcinosarcoma | 2 (22.2) |
| ŌĆāLeiomyosarcoma | 2 (22.2) |
| ŌĆāUndifferentiated uterine sarcoma | 2 (22.2) |
| ŌĆāLow-grade endometrial stromal sarcoma | 1 (11.1) |
| ŌĆāMullerian adenosarcoma | 1 (11.1) |
| ŌĆāSynovial sarcoma | 1 (11.1) |
| Disease status at the time of LEER | |
| ŌĆāPrimary disease | 3 (33.3) |
| ŌĆāRecurrent disease | 6 (66.7) |
| Initial FIGO stage | |
| ŌĆāI | 4 (44.4) |
| ŌĆāII | 1 (11.1) |
| ŌĆāIII | 2 (22.2)b) |
| ŌĆāIV | 2 (22.2) |
| Preoperative radiologic TNM stage | |
| ŌĆāT staging | |
| ŌĆāŌĆāT2b | 5 (55.6) |
| ŌĆāŌĆāT4 | 4 (44.4) |
| ŌĆāN staging | |
| ŌĆāŌĆāN0 | 6 (66.7) |
| ŌĆāŌĆāN1 | 3 (33.3) |
| ŌĆāM staging | |
| ŌĆāŌĆāM0 | 7 (77.8) |
| ŌĆāŌĆāM1 | 2 (22.2) |
| Largest radiologic tumor size prior to LEER (cm) | 10 (2-17.5) |
| Neoadjuvant chemotherapyc) | 2 (22.2)c) |
| Types of prior treatmentd) | |
| ŌĆāSystemic therapy | 5 (55.5) |
| ŌĆāRadiotherapy | 2 (22.2) |
| ŌĆāSurgery+chemotherapy | 4 (44.4) |
| ŌĆāSurgery+radiotherapy | 2 (22.2) |
| Lines of prior chemotherapy | 2 (0-5) |
| Prior systemic treatment regimend),e) | |
| ŌĆāIfosfamide-combined | 4 (44.4) |
| ŌĆāDoxorubicin only or combined | 3 (33.3) |
| ŌĆāGemcitabine-docetaxel | 1 (11.1) |
| ŌĆāPaclitaxel-carboplatin | 1 (11.1) |
| ŌĆāTargeted therapy | 1 (11.1) |
| Treatment-free interval before LEER (months) | 3.9 (1.1-38.2) |
| Best response of last treatment before LEER | |
| ŌĆāComplete response | 2 (22.2) |
| ŌĆāPartial response | 2 (22.2) |
| ŌĆāStable disease | 1 (11.1) |
| ŌĆāProgressive disease | 3 (33.3) |
| ŌĆāNot available | 1 (11.1) |
| Pelvic sidewall tumor locationd) | |
| ŌĆāInfra-iliac acetabulum | 6 (66.7) |
| ŌĆāInfra-iliac ischiopubic | 2 (22.2) |
| ŌĆāInfra-iliac sacrococcygeal | 4 (44.4) |
| Duration of follow-up (months) | 52.7 (11.4-130.4) |
LEER, laterally extended endopelvic resection; FIGO, International Federation of Gynecology and Obstetrics; TNM, The TNM classification of malignant tumors.
a) Comorbidities included hypertension (n=1, 11.1%), diabetes (n=1, 11.1%), dyslipidemia (n=2, 22.2%), thyroid disease (n=1, 11.1%), thromboembolic disease (n=1, 11.1%), and hepatitis (n=1, 11,1%), overlapping conditions included;
Table┬Ā2
| Characteristic | Value (n=9) |
|---|---|
| Organ preservation | |
| ŌĆāNo | 2 (22.2) |
| ŌĆāRectum alone | 2 (22.2) |
| ŌĆāBladder alone | 0 (0.0) |
| ŌĆāRectum and bladder both | 5 (55.6) |
| Surgical extent | |
| ŌĆāHysterectomy | 2 (22.2) |
| ŌĆāBSO | 2 (22.2) |
| ŌĆāPLND | 7 (77.8) |
| ŌĆāPALND | 7 (77.8) |
| ŌĆāCystectomy | 4 (44.4) |
| ŌĆāVaginectomy | 4 (44.4) |
| ŌĆāInternal iliac vessel resection | 9 (100.0) |
| ŌĆāPelvic sidewall muscle resection | 8 (88.9) |
| ŌĆāŌĆāObturator internus muscle | 6 (66.7) |
| ŌĆāŌĆāIliococcygeus muscle | 3 (33.3) |
| ŌĆāŌĆāPubococcygeus muscle | 5 (55.6) |
| ŌĆāŌĆāCoccygeus muscle | 3 (33.3) |
| ŌĆāUreter ligation and resection | 8 (88.9) |
| ŌĆāVulvectomy (perineum) | 4 (44.4) |
| ŌĆāBowel resection | 5 (55.6) |
| ŌĆāIleal conduit | 4 (44.4) |
| ŌĆāColostomy | 2 (22.2) |
| ŌĆāOthersa) | 5 (55.5) |
| Pathologic tumor size (cm) | 9.0 (1.8-19.0) |
| Pathologic extent | |
| ŌĆāUterus | 2 (22.2) |
| ŌĆāVagina | 4 (44.4) |
| ŌĆāPerineum | 0 (0.0) |
| ŌĆāBladder and urethra | 7 (77.8) |
| ŌĆāAnus and rectum | 5 (55.6) |
| ŌĆāPelvic sidewall muscle | 7 (77.8) |
| ŌĆāInternal iliac vessel | 6 (66.7) |
| Tumor grade | |
| ŌĆāLow-grade | 1 (11.1) |
| ŌĆāHigh-grade | 8 (88.9) |
| Residual tumor | |
| ŌĆāR0 | 8 (88.9) |
| ŌĆāR1 | 1 (11.1) |
| Operation time (minutes) | 300 (135-1,320) |
| Estimated blood loss (mL) | 1,600 (300-22,300) |
| Transfusion | |
| ŌĆāRBC | 3 (0-42) |
| ŌĆāFFP | 0 (0-34) |
| ŌĆāPC | 0 (0-24) |
| Postoperative ICU admission (days) | 1 (0-8) |
| Postoperative complications (according to MSKCC grading system) | |
| ŌĆāGastrointestinal system (ileus) | |
| ŌĆāŌĆāGrade 1/2 | 2 (22.2) |
| ŌĆāŌĆāGrade 3/4 | 0 (0.0) |
| ŌĆāGenitourinary system (urinary incontinence, voiding difficulty) | |
| ŌĆāŌĆāGrade 1/2 | 2 (22.2) |
| ŌĆāŌĆāGrade 3/4 | 0 (0.0) |
| ŌĆāInfection | |
| ŌĆāŌĆāGrade 1/2 | 0 (0.0) |
| ŌĆāŌĆāGrade 3/4 | 3 (33.3) |
| ŌĆāNervous system | |
| ŌĆāŌĆāGrade 1/2 | 4 (44.4) |
| ŌĆāŌĆāGrade 3/4 | 0 (0.0) |
| Pelvic pain severity | |
| ŌĆāPreoperative NRS | 4 (0-7) |
| ŌĆāPostoperative NRS | 2 (1-3) |
| ŌĆāPreoperative MME (mg/day) | 0 (0-105) |
| ŌĆāPostoperative MME (mg/day) | 0 (0-15) |
| Postoperative adjuvant treatment | |
| ŌĆāNo adjuvant treatment | 3 (33.3) |
| ŌĆāConcurrent chemoradiation followed by hormone therapy | 1 (11.1) |
| ŌĆāChemotherapy | 4 (44.4) |
| ŌĆāConcurrent chemoradiation | 1 (11.1) |
| Treatment response at postoperative 3 months | |
| ŌĆāComplete response | 4 (44.4) |
| ŌĆāPartial response | 0 (0.0) |
| ŌĆāStable disease | 0 (0.0) |
| ŌĆāProgression or recurrence | 4 (44.4) |
| ŌĆāNot assessable | 1 (11.1) |
| Recurrence | 6 (66.7) |
| Death | 5 (55.6) |
LEER, laterally extended endopelvic resection; BSO, bilateral-salpingo-oophorectomy; PLND, pelvic lymph node dissection; PALND, para-aortic lymph node dissection; RBC, red blood cell; FFP, fresh frozen plasma; PC, platelet concentrate; ICU, intensive care unit; MSKCC, Memorial Sloan Kettering Cancer Center; NRS, numeric rating scale; MME, morphine milligram equivalents.
Table┬Ā3
LEER, laterally extended endopelvic resection; FIGO, International Federation of Gynecology and Obstetrics; TNM, the TNM classification of malignant tumors; TFI, treatment free survival; PFS, progression free survival; TRS, treatment-related survival; OS, overall survival; UC, uterine cancer; CCRT, concurrent chemoradiation; CR, complete response; AJCC, American Joint Committee of Cancer; OC, ovarian cancer; PD, progressive disease; FU, follow up; Lt, left; Rt, right; NA, not available.
References
- TOOLS