 |
 |
- Search
| Obstet Gynecol Sci > Volume 65(6); 2022 > Article |
|
Abstract
Objective
Methods
Results
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported. Ki Hoon Ahn and Geum-Joon Cho have been an Editorial Board of Obstetrics & Gynecology Science; however, they are not involved in the peer reviewer selection, evaluation, or decision process of this article. Otherwise, no other potential conflicts of interest relevant to this article were reported.
Ethical approval
This retrospective cohort study was approved by the Institutional Review Board (IRB) of the Korea University Anam Hospital on August 19, 2019 (2019AN0354).
Fig.┬Ā1
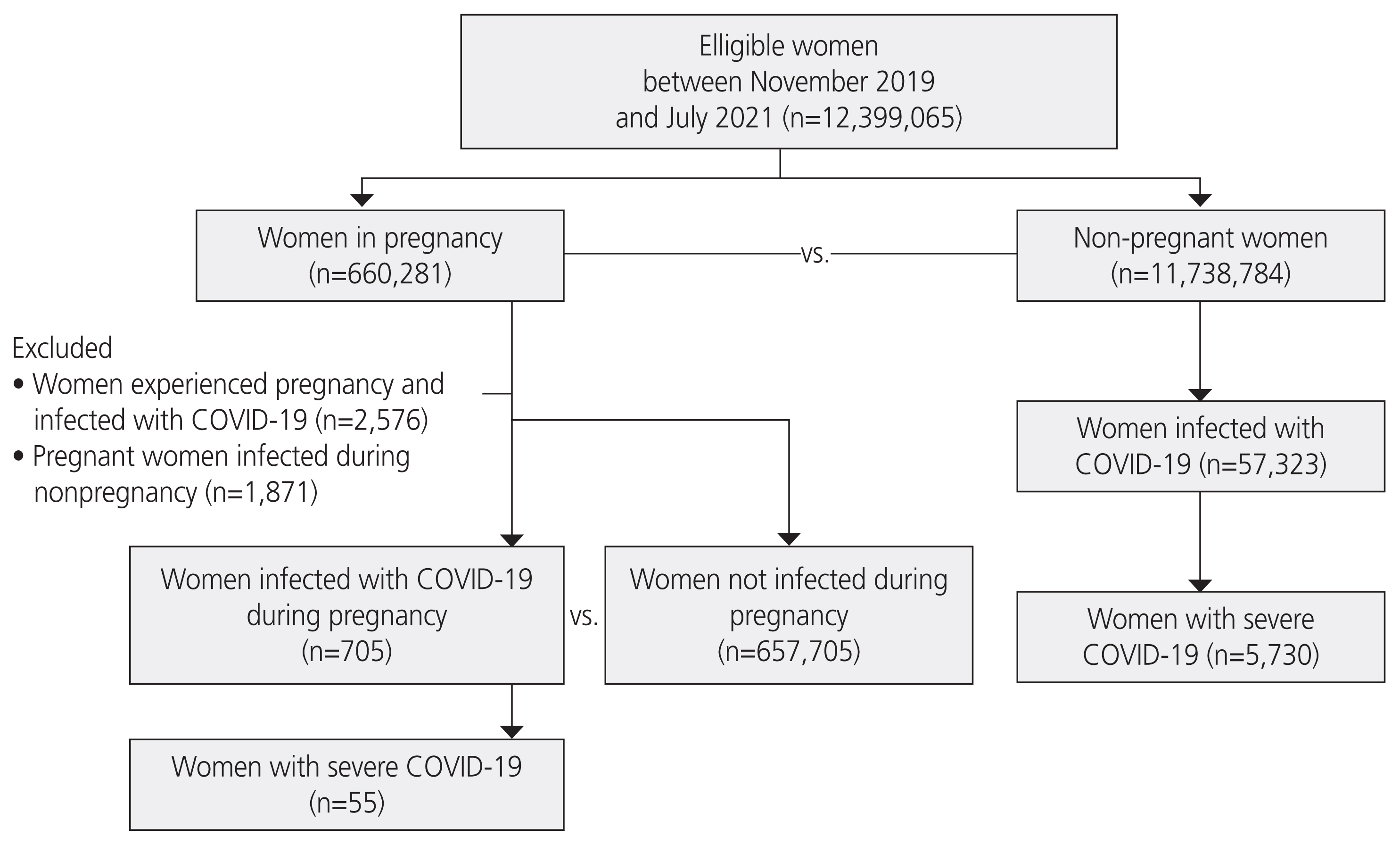
Table┬Ā1
| Total women (n=12,397,194a)) | |
|---|---|
| Pregnancy, yes | |
| ŌĆāCOVID-19, yes | 705 (0.11%) |
| ŌĆāCOVID-19, no | 657,705 (99.89%) |
| Total | 658,410 (5.31% of total women) |
| Pregnancy, no | |
| ŌĆāCOVID-19, yes | 57,323 (0.49%) |
| ŌĆāCOVID-19, no | 11,681,461 (99.51%) |
| Total | 11,738,784 (94.68% of total women) |
Table┬Ā2-1
| Variable | Diagnosis | Pregnant women without COVID-19 (n=657,705) | Pregnant women with COVID-19 (n=705) | P-value |
|---|---|---|---|---|
| O00 | Ectopic pregnancy | 4,350 (0.66) | 5 (0.71) | >0.9999 |
| O01 | Hydatidiform mole | 879 (0.13) | 0 (0.00) | >0.9999 |
| O02 | Other abnormal products of conception | 60,754 (9.24) | 94 (13.33) | 0.0002a) |
| O03 | Spontaneous abortion | 10,838 (1.65) | 17 (2.41) | 0.1490 |
| O04 | Complications following (induced) termination of pregnancy | 754 (0.11) | 1 (0.14) | 0.5548 |
| O05 | Other abortion | 1,093 (0.17) | 0 (0.00) | 0.6365 |
| O06 | Unspecified abortion | 1,813 (0.28) | 3 (0.43) | 0.4508 |
| O07 | Failed attempted termination of pregnancy | 29 (0.00) | 0 (0.00) | >0.9999 |
| O08 | Complications following ectopic and molar pregnancy | 4,902 (0.75) | 3 (0.43) | 0.5052 |
| O10 | Pre-existing hypertension complicating pregnancy, childbirth and the puerperium | 2,946 (0.45) | 3 (0.43) | >0.9999 |
| O11 | Pre-existing hypertensive disorder with superimposed proteinuria | 770 (0.12) | 1 (0.14) | 0.5624 |
| O12 | Gestational (pregnancy-induced) edema and proteinuria without hypertension | 143,983 (21.89) | 91 (12.91) | <0.0001a) |
| O13 | Gestational (pregnancy-induced) hypertension without significant proteinuria | 17,210 (2.62) | 13 (1.84) | 0.2433 |
| O14 | Gestational (pregnancy-induced) hypertension with significant proteinuria | 9,064 (1.38) | 8 (1.13) | 0.6948 |
| O15 | Eclampsia | 233 (0.04) | 0 (0.00) | >0.9999 |
| O16 | Unspecified maternal hypertension | 6,881 (1.05) | 9 (1.28) | 0.6777 |
| O20 | Hemorrhage in early pregnancy | 184,568 (28.06) | 206 (29.22) | 0.5211 |
| O21 | Excessive vomiting in pregnancy | 128,205 (19.49) | 144 (20.43) | 0.5638 |
| O22 | Venous complications in pregnancy | 138,062 (20.99) | 100 (14.18) | <0.0001a) |
| O23 | Infection of genitourinary tract in pregnancy | 153,156 (23.29) | 114 (16.17) | <0.0001a) |
| O24 | Diabetes mellitus in pregnancy | 231,823 (35.25) | 184 (26.1) | <0.0001a) |
| O25 | Malnutrition in pregnancy | 442 (0.07) | 1 (0.14) | 0.3780 |
| O26 | Maternal care for other conditions predominantly related to pregnancy | 33,796 (5.14) | 27 (3.83) | 0.1368 |
| O28 | Abnormal findings on antenatal screening of mother | 17,564 (2.67) | 12 (1.70) | 0.1396 |
| O29 | Complications of anesthesia during pregnancy | 92 (0.01) | 0 (0.00) | >0.9999 |
| O30 | Multiple gestation | 23,485 (3.57) | 31 (4.4) | 0.2800 |
| O31 | Complications specific to multiple gestation | 1,587 (0.24) | 1 (0.14) | >0.9999 |
| O32 | Maternal care for known or suspected malpresentation of fetus | 39,625 (6.02) | 29 (4.11) | 0.0401a) |
| O33 | Maternal care for known or suspected disproportion | 71,341 (10.85) | 28 (3.97) | <0.0001a) |
| O34 | Maternal care for known or suspected abnormality of pelvic organs | 126,536 (19.24) | 119 (16.88) | 0.1234 |
| O35 | Maternal care for known or suspected fetal abnormality and damage | 17,257 (2.62) | 13 (1.84) | 0.2392 |
| O36 | Maternal care for other known or suspected fetal problems | 41,246 (6.27) | 30 (4.26) | 0.0332a) |
| O30 | Multiple gestation | 23,485 (3.57) | 31 (4.4) | 0.2800 |
| O31 | Complications specific to multiple gestation | 1,587 (0.24) | 1 (0.14) | >0.9999 |
| O32 | Maternal care for known or suspected malpresentation of fetus | 39,625 (6.02) | 29 (4.11) | 0.0401a) |
| O33 | Maternal care for known or suspected disproportion | 71,341 (10.85) | 28 (3.97) | <0.0001a) |
| O34 | Maternal care for known or suspected abnormality of pelvic organs | 126,536 (19.24) | 119 (16.88) | 0.1234 |
| O35 | Maternal care for known or suspected fetal abnormality and damage | 17,257 (2.62) | 13 (1.84) | 0.2392 |
| O36 | Maternal care for other known or suspected fetal problems | 41,246 (6.27) | 30 (4.26) | 0.0332a) |
| O40 | Polyhydramnios | 5,338 (0.81) | 4 (0.57) | 0.6717 |
| O41 | Other disorders of amniotic fluid and membranes | 28,652 (4.36) | 23 (3.26) | 0.1835 |
| O42 | Premature rupture of membranes | 101,828 (15.48) | 67 (9.5) | 0.0000a) |
| O43 | Placental disorders | 3,597 (0.55) | 1 (0.14) | 0.1974 |
| O44 | Placenta previa | 19,580 (2.98) | 20 (2.84) | 0.9140 |
| O45 | Premature separation of placenta (abruptio placentae) | 2,393 (0.36) | 3 (0.43) | 0.7466 |
| O46 | Antepartum hemorrhage, NEC | 18,190 (2.77) | 12 (1.7) | 0.1082 |
| O47 | False labor | 73,269 (11.14) | 45 (6.38) | 0.0001a) |
| O48 | Prolonged pregnancy | 3,062 (0.47) | 1 (0.14) | 0.3936 |
| O60 | Preterm delivery | 140,953 (21.43) | 124 (17.59) | 0.0147a) |
| O61 | Failed induction of labor | 18,538 (2.82) | 13 (1.84) | 0.1473 |
| O62 | Abnormalities of forces of labor | 11,982 (1.82) | 8 (1.13) | 0.2215 |
| O63 | Long labor | 17,483 (2.66) | 6 (0.85) | 0.0042a) |
| O64 | Obstructed labor due to malposition and malpresentation of fetus | 3,686 (0.56) | 1 (0.14) | 0.1993 |
| O65 | Obstructed labor due to maternal pelvic abnormality | 36,981 (5.62) | 15 (2.13) | 0.0001a) |
| O66 | Other obstructed labor | 12,729 (1.94) | 4 (0.57) | 0.0038a) |
| O67 | Labor and delivery complicated by intrapartum hemorrhage, NEC | 4,923 (0.75) | 0 (0.00) | 0.0128a) |
| O68 | Labor and delivery complicated by fetal stress (distress) | 23,397 (3.56) | 16 (2.27) | 0.0812 |
| O69 | Labor and delivery complicated by umbilical cord complications | 3,336 (0.51) | 4 (0.57) | 0.7862 |
| O70 | Perineal laceration during delivery | 42,428 (6.45) | 15 (2.13) | <0.0001a) |
| O71 | Other obstetric trauma | 11,043 (1.68) | 3 (0.43) | 0.0048a) |
| O72 | Postpartum hemorrhage | 62,532 (9.51) | 23 (3.26) | <0.0001a) |
| O73 | Retained placenta and membranes, without hemorrhage | 2,076 (0.32) | 1 (0.14) | 0.7320 |
| O74 | Complications of anesthesia during labor and delivery | 361 (0.05) | 0 (0.00) | >0.9999 |
| O75 | Other complications of labor and delivery, NEC | 3,852 (0.59) | 0 (0.00) | 0.0408a) |
| O80 | Single spontaneous delivery | 146,224 (22.23) | 72 (10.21) | <0.0001a) |
| O81 | Single delivery by forceps and vacuum extractor | 21,636 (3.29) | 8 (1.13) | 0.0019a) |
| O82 | Single delivery by cesarean section | 222,780 (33.87) | 157 (22.27) | <0.0001a) |
| O83 | Other assisted single delivery | 32,734 (4.98) | 18 (2.55) | 0.0041a) |
| O84 | Multiple delivery | 7,494 (1.14) | 4 (0.57) | 0.2092 |
| O85 | Puerperal sepsis | 595 (0.09) | 1 (0.14) | 0.4721 |
| O86 | Other puerperal infections | 65,996 (10.03) | 19 (2.7) | 0.0000a) |
| O87 | Venous complications during puerperium | 61,483 (9.35) | 36 (5.11) | 0.0001a) |
| O88 | Obstetric embolism | 132 (0.02) | 0 (0.00) | >0.9999 |
| O89 | Complications of anesthesia during the puerperium | 559 (0.08) | 0 (0.00) | >0.9999 |
| O90 | Complications of the puerperium, NEC | 29,280 (4.45) | 10 (1.42) | 0.0001a) |
| O91 | Infections of the breast associated with childbirth | 17,045 (2.59) | 5 (0.71) | 0.0025a) |
| O92 | Other disorders of the breast and lactation associated with childbirth | 8,306 (1.26) | 0 (0.00) | 0.0003a) |
| O94 | Sequelae of complication of pregnancy, childbirth and the puerperium | 237,195 (36.06) | 218 (30.92) | 0.0051a) |
Table┬Ā2-2
| Variable | Pregnant women without COVID-19 (n=657,705) | Pregnant women with COVID-19 (n=705) | P-value |
|---|---|---|---|
| Preterm birth | 98,227 (14.93) | 76 (10.78) | 0.0024a) |
| Cesarean section | 232,849 (35.40) | 133 (18.87) | <0.0001a) |
Table┬Ā2-3
| Total women (n=12,397,194a)) | |
|---|---|
| Pregnancy, yes | |
| ŌĆāSevere COVID-19, yes | 55 (7.80) |
| ŌĆāSevere COVID-19, no | 650 (92.20) |
| Pregnancy, no | |
| ŌĆāSevere COVID-19, yes | 5,730 (10.00) |
| ŌĆāSevere COVID-19, no | 51,593 (90.00) |
Table┬Ā3-1
| Variable | Diagnosis | Pregnant women without COVID-19 (n=336,114) | Pregnant women with COVID-19 (n=139) | P-value |
|---|---|---|---|---|
| P00 | Fetus and newborn affected by maternal conditions that may be unrelated to present pregnancy | 9,852 (2.93) | 22 (15.83) | <0.0001a) |
| P01 | Fetus and newborn affected by maternal complications of pregnancy | 9,859 (2.93) | 4 (2.88) | 0.9999 |
| P02 | Fetus and newborn affected by maternal complications of placenta, cord and membranes | 2,549 (0.76) | 0 (0.00) | 0.6312 |
| P03 | Fetus and newborn affected by other complications of labor and delivery | 7,296 (2.17) | 10 (7.19) | <0.0001a) |
| P04 | Fetus and newborn affected by noxious influences transmitted via the placenta or breast milk | 1,530 (0.46) | 0 (0.00) | >0.9999 |
| P05 | Slow fetal growth and fetal malnutrition | 3,392 (1.01) | 3 (2.16) | 0.1666 |
| P07 | Disorders related to short gestation and low birth weight, NEC | 21,796 (6.48) | 14 (10.07) | 0.1224 |
| P08 | Disorders related to long gestation and high birth weight | 4,429 (1.32) | 1 (0.72) | >0.9999 |
| P10 | Intracranial laceration and hemorrhage due to birth injury | 101 (0.03) | 0 (0.00) | >0.9999 |
| P11 | Other birth injuries to central nervous system | 30 (0.01) | 0 (0.00) | >0.9999 |
| P12 | Birth injury to scalp | 3,114 (0.93) | 4 (2.88) | 0.0412a) |
| P13 | Birth injury to skeleton | 3,405 (1.01) | 1 (0.72) | >0.9999 |
| P14 | Birth injury to peripheral nervous system | 50 (0.01) | 0 (0.00) | >0.9999 |
| P15 | Other birth injuries | 90 (0.03) | 0 (0.00) | >0.9999 |
| P20 | Intrauterine hypoxia | 980 (0.29) | 1 (0.72) | 0.3338 |
| P21 | Birth asphyxia | 653 (0.19) | 0 (0.00) | >0.9999 |
| P22 | Respiratory distress of newborn | 27,571 (8.2) | 17 (12.23) | 0.1152 |
| P23 | Congenital pneumonia | 1,193 (0.35) | 0 (0.00) | >0.9999 |
| P24 | Neonatal aspiration syndromes | 12,067 (3.59) | 5 (3.6) | >0.9999 |
| P25 | Interstitial emphysema and related conditions originating in the perinatal period | 715 (0.21) | 0 (0.00) | >0.9999 |
| P26 | Pulmonary hemorrhage originating in the perinatal period | 88 (0.03) | 0 (0.00) | >0.9999 |
| P27 | Chronic respiratory disease originating in the perinatal period | 654 (0.19) | 0 (0.00) | >0.9999 |
| P28 | Other respiratory conditions originating in the perinatal period | 8,980 (2.67) | 4 (2.88) | 0.7899 |
| P29 | Cardiovascular disorders originating in the perinatal period | 1,992 (0.59) | 0 (0.00) | >0.9999 |
| P35 | Congenital viral diseases | 710 (0.21) | 0 (0.00) | >0.9999 |
| P36 | Bacterial sepsis of newborn | 4,657 (1.39) | 2 (1.44) | 0.7192 |
| P37 | Other congenital infectious and parasitic diseases | 235 (0.07) | 0 (0.00) | >0.9999 |
| P38 | Omphalitis of newborn with or without mild hemorrhage | 29,331 (8.73) | 16 (11.51) | 0.3113 |
| P39 | Other infections specific to the perinatal period | 25,291 (7.52) | 15 (10.79) | 0.1940 |
| P50 | Fetal blood loss | 67 (0.02) | 0 (0.00) | >0.9999 |
| P51 | Umbilical hemorrhage of newborn | 1,475 (0.44) | 1 (0.72) | 0.4575 |
| P52 | Intracranial nontraumatic hemorrhage of fetus and newborn | 1,559 (0.46) | 1 (0.72) | 0.4761 |
| P53 | Hemorrhagic disease of fetus and newborn | 1,016 (0.30) | 0 (0.00) | >0.9999 |
| P54 | Other neonatal hemorrhages | 1,025 (0.30) | 2 (1.44) | 0.0680 |
| P55 | Hemolytic disease of fetus and newborn | 1,132 (0.34) | 0 (0.00) | >0.9999 |
| P56 | Hydrops fetalis due to hemolytic disease | 5 (0.00) | 0 (0.00) | >0.9999 |
| P57 | Kernicterus | 58 (0.02) | 0 (0.00) | >0.9999 |
| P58 | Neonatal jaundice due to other excessive hemolysis | 1,128 (0.34) | 2 (1.44) | 0.0801 |
| P59 | Neonatal jaundice from other and unspecified causes | 133,504 (39.72) | 57 (41.01) | 0.8232 |
| P60 | Disseminated intravascular coagulation of fetus and newborn | 308 (0.09) | 0 (0.00) | >0.9999 |
| P61 | Other perinatal hematological disorders | 2,425 (0.72) | 1 (0.72) | >0.9999 |
| P70 | Transitory disorders of carbohydrate metabolism specific to fetus and newborn | 25,904 (7.71) | 9 (6.47) | 0.6999 |
| P71 | Transitory neonatal disorders of calcium and magnesium metabolism | 3,990 (1.19) | 1 (0.72) | >0.9999 |
| P72 | Other transitory neonatal endocrine disorders | 6,748 (2.01) | 6 (4.32) | 0.1015 |
| P74 | Other transitory neonatal electrolyte and metabolic disturbances | 3,552 (1.06) | 3 (2.16) | 0.1828 |
| P75 | Meconium ileus | 3 (0.00) | 0 (0.00) | >0.9999 |
| P76 | Other intestinal obstruction of newborn | 256 (0.08) | 0 (0.00) | >0.9999 |
| P77 | Necrotizing enterocolitis of fetus and newborn | 437 (0.13) | 0 (0.00) | >0.9999 |
| P78 | Other perinatal digestive system disorders | 3,891 (1.16) | 2 (1.44) | 0.6773 |
| P80 | Hypothermia of newborn | 154 (0.05) | 0 (0.00) | >0.9999 |
| P81 | Other disturbances of temperature regulation of newborn | 1,661 (0.49) | 2 (1.44) | 0.1512 |
| P83 | Other conditions of integument specific to fetus and newborn | 12,061 (3.59) | 3 (2.16) | 0.4948 |
| P90 | Convulsions of newborn | 659 (0.20) | 0 (0.00) | >0.9999 |
| P91 | Other disturbances of cerebral status of newborn | 1,130 (0.34) | 0 (0.00) | >0.9999 |
| P92 | Feeding problems of newborn | 13,302 (3.96) | 4 (2.88) | 0.6651 |
| P93 | Reactions and intoxications due to drugs administered to fetus and newborn | 1 (0.00) | 0 (0.00) | >0.9999 |
| P94 | Disorders of muscle tone of newborn | 117 (0.03) | 0 (0.00) | >0.9999 |
| P95 | Fetal death of unspecified cause | 0 (0.00) | 0 (0.00) | >0.9999 |
| P96 | Other conditions originating in the perinatal period | 5,060 (1.51) | 2 (1.44) | >0.9999 |
Table┬Ā3-2
| Variable | Pregnant women without COVID-19 (n=336,114) | Pregnant women with COVID-19 (n=139) | P-value |
|---|---|---|---|
| Low birth weight | |||
| ŌĆā1,000-1,249 g | 114 (0.03) | 0 (0.00) | >0.9999 |
| ŌĆā1,250-1,499 g | 403 (0.12) | 0 (0.00) | >0.9999 |
| ŌĆā1,500-1,749 g | 993 (0.30) | 0 (0.00) | >0.9999 |
| ŌĆā1,750-1,999 g | 2,173 (0.65) | 1 (0.72) | 0.5942 |
| ŌĆā2,000-2,499 g | 8,420 (2.51) | 6 (4.32) | 0.2737 |
| ŌĆāEtc. | 112 (0.03) | 0 (0.00) | >0.9999 |
| ŌĆāUnknown weight | 1,513 (0.45) | 0 (0.00) | >0.9999 |
| ŌĆāTotal | 13,728 (4.08) | 7 (5.04) | 0.7245 |
| Macrosomia | 4,429 (1.32) | 1 (0.72) | >0.9999 |
| NICU | 25,971 (7.73) | 26 (18.71) | <0.0001a) |
Table┬Ā4-1
| Moderna | Janssen | AstraZeneca | Pfizer | |
|---|---|---|---|---|
| Vaccination during pregnancy | ||||
| ŌĆāNo | 34,504 | 7,418 | 14,634 | 195,131 |
| ŌĆāYes | 1,734 | 439 | 1,135 | 10,132 |
Table┬Ā4-2
Table┬Ā5
O00-O08, pregnancy with abortive outcome; O10-O16, edema, proteinuria, and hypertensive disorders in pregnancy, childbirth, and puerperium; O20-O29, other maternal disorders predominantly related to pregnancy; O30-O48, maternal care related to the fetus and amniotic cavity and possible delivery problems; O60-O75, complications of labor and delivery; O80-O84, encounter for delivery; O85-O92, complications predominantly related to puerperium; O94-O99, other obstetric conditions, not elsewhere classified.
References
- TOOLS





















