|
 |
- Search
| Obstet Gynecol Sci > Volume 64(1); 2021 > Article |
|
Abstract
Dysmenorrhea is one of the well-established problems among women of reproductive age and can have adverse effects on the quality of life of the individual. Some studies suggest a relationship between vitamin D (Vit D) and calcium deficiency and the emergence of early dysmenorrhea. Accordingly, a systematic study was performed to investigate the role of calcium and Vit D in the relief of primary dysmenorrhea. A systematic literature search was performed in PubMed, Web of Science, Scopus, Science Direct, and Google Scholar for papers published between 2010 and 2020. The Consolidated Standards of Reporting Trials and Strengthening the Reporting of Observational Studies in Epidemiology checklists were used to assess the quality of the studies. The risk of bias was assessed using the Cochrane risk-of-bias assessment tool. Low calcium levels lead to an increase in uterine muscle contraction and can cause pain after decreased uterine blood flow. Furthermore, low levels of Vit D can increase primary dysmenorrhea by increasing prostaglandin production or reducing intestinal calcium absorption. That being the case, Vit D and calcium intake can be effective in reducing the severity of primary dysmenorrhea and in reducing the rate of analgesic use. Low levels of Vit D and calcium are inversely related to the severity of primary dysmenorrhea, and Vit D and calcium intake can reduce the severity of primary dysmenorrhea and its associated systemic symptoms. Therefore, the use of calcium and Vit D supplements can be recommended to relieve dysmenorrhea.
Dysmenorrhea or menstrual cramps is one of the most common problems among women of reproductive age [1,2] and can have adverse effects on the health and quality of life of the individual and the community [3]. More than half of the women experience this pain on the first or second day of menstruation [4]. However, its usual onset is between 6 and 12 months from the age of menarche [5]. Dysmenorrhea occurs as both primary and secondary types, of which the primary type includes lower abdominal pain early in menstruation and the absence of any known pelvic pathology [6]. The prevalence of primary dysmenorrhea in the world is reported to be between 45% and 95% [7], especially in Nigeria, 45.5% [5]; China, 51.1% [6]; Ibadan in Nigeria, 73% [8]; Ghana, 68.1% [9]; Japan, 72.8% [10]; Kuwait, 85.6% [11]; the city of Paraco in the Republic of Benin, 78.3% [12]; Ankara, 84% [13]; Egypt, 93% [14]; and, in a study, at the University of Isfahan, approximately, 89.1% [15].
Although the mechanism of primary dysmenorrhea has not been unraveled, it is thought to be due to an increase in the production of prostaglandins and leukotrienes from the myometrium. At the end of the ovulation phase, fatty acids are produced and accumulated in the cell membrane. At the end of the cycle, decreased progesterone levels send signals for the onset of menstruation and the release of fatty acids including arachidonic acid as a precursor to the production of dinoprostone (prostaglandin E2), carboprost (prostaglandin F2α), and leukotrienes. These substances cause contractions of the myometrium and induce pain during menstruation [4].
Dysmenorrhea is one of the most prevalent problems among premenopausal women, with many social consequences such as absenteeism from work and school. It should be noted that dysmenorrhea has a negative effect on a person’s relationships with family members and society, as well as on a person’s quality of life [11]. In addition, decreased sleep quality and physical activity, and poor mood are among the consequences of dysmenorrhea [16]. Various studies have shown that 24.1% of students with dysmenorrhea have problems in concentration in the classroom, 14.9% in social activities, 6.2% in sports activities, 16.79% of class attendance, and 11.60% had problems in all of the above. Of them, 1.8% were excluded from attending and concentrating in the classroom [8]. Furthermore, dysmenorrhea is associated with huge economic consequences. In the United States of America, the economic burden of dysmenorrhea is estimated at 600 million working hours, or $200 billion [17], and in Japan, it is estimated at $4.2 million per year [15].
In this regard, great effort has been made to reduce dysmenorrhea and its consequences, and the possible role of certain substances and eating habits in the incidence of dysmenorrhea has been raised [18]. Metabolism and absorption of vitamins and minerals can play a major role in the treatment and extent of dysmenorrhea. Calcium regulates the ability of muscle cells to respond to nerve stimulation and acts as a stabilizing agent. Decreased calcium levels can lead to muscle spasms and contractions. Calciferol, or the active form of vitamin D (Vit D), also plays a large role in regulating prostaglandin levels [19]. In other words, calcium homeostasis, under the influence of Vit D levels, can effectively relieve dysmenorrhea [20]. In this aspect, an inverse relationship was observed between dairy consumption and dysmenorrhea. Thus, with the increased consumption of dairy products containing the highest amount of calcium, the incidence of dysmenorrhea decreased and this role further increased the use of calcium in reducing dysmenorrhea [21]. In another study, 1,000 mg of calcium alone daily effectively reduced pain intensity; however, these results were not observed with 1,000 mg of calcium with 5,000 units of Vit D daily [19]. Cinnamon has also been shown to be significantly more effective than Vit E and Vit D in relieving the severity of dysmenorrhea pain [20]. In a study in Iran, no significant relationship was observed between serum Vit D levels and mean pain intensity in primary dysmenorrhea [22].
Given the negative effects of primary dysmenorrhea on the mental and social health of the affected women and those around them and the material costs imposed on governments, it seems reasonable to strive to find an effective solution to reduce the incidence or the consequences of the problem. In this regard, it is quintessential to pay particular attention to the role of calcium and Vit D in reducing the incidence of dysmenorrhea. Accordingly, this study sought to investigate the role of Vit D and calcium in relieving primary dysmenorrhea.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed in this study. The study contains 27 items related to the systematic review and meta-analysis and includes abstracts, methods, results, discussions, and financial resources. A literature search was conducted in PubMed, Web of Science, Scopus, Science Direct, Google Scholar, and Science Direct between 2010 and 2020, using the following keywords (Table 1).
The inclusion criteria are as follows: primary dysmenorrhea, low serum Vit D levels, examination of the role or effect of calcium and Vit D in primary dysmenorrhea, being a non-smoker or non-alcoholic, no abnormal vaginal and cervical secretions, no stressful events, no history of uterine disorders (fibroids, duodenal ulcers, polyps, endometrial hypertrophy and endometriosis) and ovarian disorders (ovarian cysts and polycystic ovaries), lack of calcium and Vit D supplementation, and regular menstrual cycles in the last 6 months.
The exclusion criteria are as follows: history of gastrointestinal upset, heart and kidney disease, history of mental illness and drug use, pregnancy, use of hormonal contraceptives, sensitivity to Vit D or calcium, and unwillingness to participate in studies. PICO criteria are shown in Table 2.
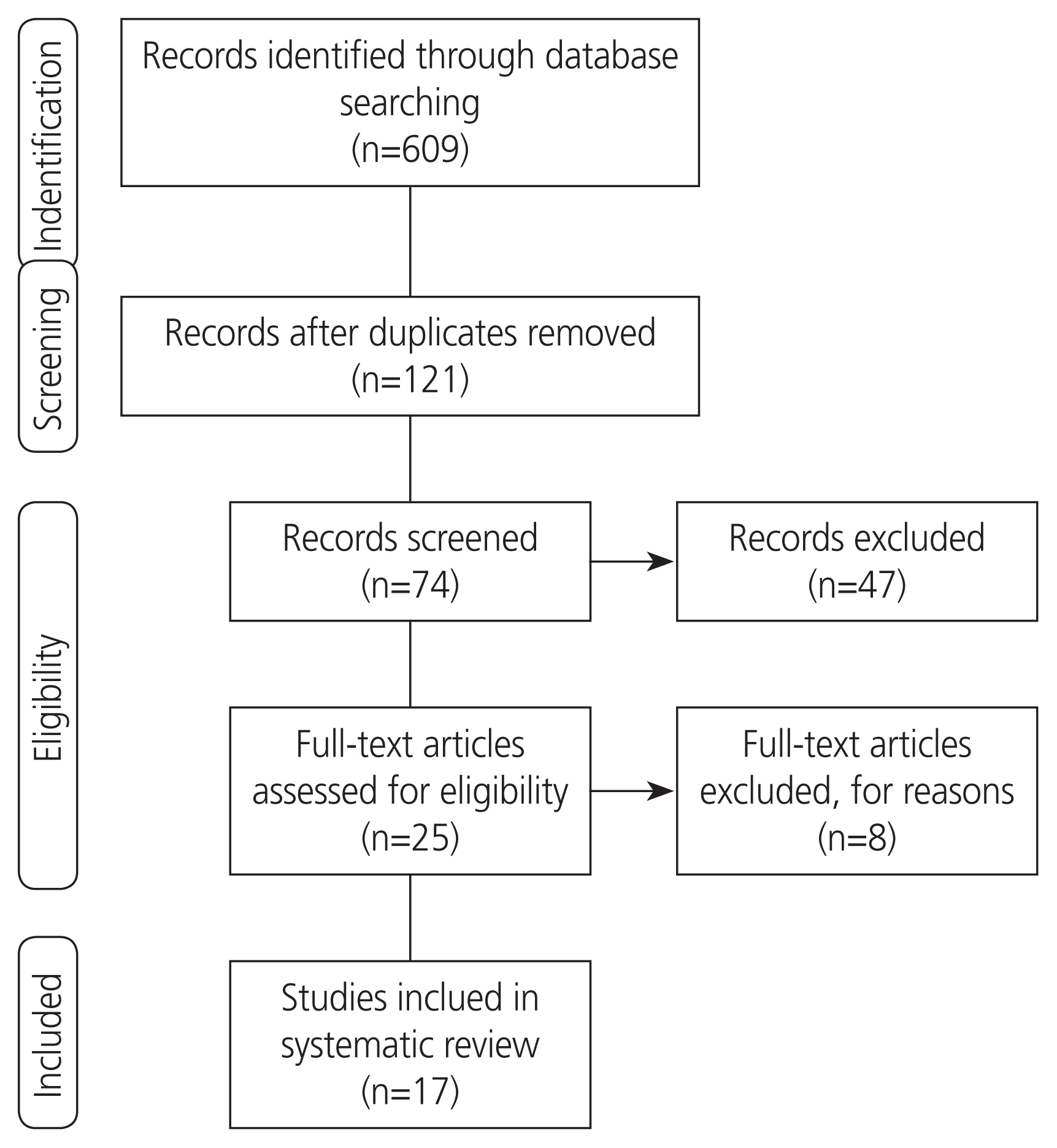
The initial search yielded 609 results. The eligibility of these articles was independently evaluated by 2 authors, and any disagreements were resolved by consensus. In the first stage, 488 articles were excluded for irrelevance or duplication. After reviewing the titles and abstracts of the remaining articles, 47 more papers were excluded. On evaluation of the full texts, 8 out of the remaining 25 articles were excluded for ineligibility. Finally, a total of 17 eligible articles were reviewed (Fig. 1).
The Consolidated Standards of Reporting Trials (CONSORT), Strengthening the Reporting of Observational Studies in Epidemiology (STROBE), and Cochrane risk-of-bias statements were applied to evaluate the quality of the studies. The CONSORT statement comprises a 25-item checklist. The checklist items focus on reporting how the trial is designed, analyzed, and interpreted. The STROBE statement, as an authoritative tool, consists of a 22-item checklist. The checklist items concentrate on reporting or evaluating different sections of observational studies [23,24]. The STROBE checklist consists of 6 general sections under the following headings: title and abstract, introduction, methods, results, discussion, and other information. In the Methods section of the STROBE statement, it is recommended that the study design be specified and mentioned at the beginning of the implementation method, and the place and time of the study, the duration of exposure and follow-up, and how to collect data should be clearly stated. Inclusion criteria, selection and follow-up criteria, inclusion criteria, how to select people, the population to which the participants belong, and the method of following people during the study period, are important factors that should be addressed in the study method, along with matching and their number to determine the outcome, exposure, distortion, opposite effects. In this way, the outcome under study, the intended exposure and the factors that may play a distorting role in the study or interact with the exposure and outcome under review, should be well defined and specified. Explanations on how to measure data and how to measure outcome or exposure should be provided. CONSORT contains 6 general parts that are: title and summary, introduction, method of implementation, results, discussion, and other information. Each of these 6 general parts is in the form of several subparts. The title is explained, followed by the others which are: background and objectives (introduction); trial design, participants, interventions, consequences, sample size, randomization that includes sequence creation, method of hiding the allocation of participants and execution, course building, and statistical methods (the method execution section); flow of participants, morbidity, information basis, the number of people analyzed, consequences and estimates, sub-analyses, and risks (part of the results); limitations, generalizability, and interpretation (discussion section); and registration of the trial, instructions, and financing (other parts of the information).
The risk of bias was assessed by using the Cochrane risk-of-bias assessment tool. The risks identified were compared, and disagreements were resolved by consensus (Table 3).
Two authors independently performed the study selection and validity assessment and resolved any disagreements by consulting a third researcher. The first author’s name, publication year, country, study design, sample size, age, menarche age, body mass index (BMI), duration of each menstrual dysmenorrhea, duration of menstruation cycle, intervention, control, pain intensity, serum Vit D level, tools, definition of tools, results, and quality score were extracted.
Out of a total of 17 related studies, 3 [22,25,26] had a cross-sectional design, 1 [24] had a case-control design, 2 [27,28] had a semi-experimental design, and 11 [19,29-38] were randomized controlled trials. Furthermore, 10 studies [19,22,25,28,29,32,33,35-37] were performed in Iran, 3 [18,27,30] in Turkey, and 1 [26] in Jordan, 1 [31] in India, 1 [35] in Italy, and 1 [31] in Saudi Arabia. A total of 2,828 women had participated in the study, and their age ranged from 12 to 30 years old, and the majority had a normal BMI. Age at menarche of the participants was mostly normal. Out of 4 studies, 2 [22,26] did not show any relationship between serum Vit D levels and the intensity of primary dysmenorrhea. In contrast, 2 studies [18,25] reported that there was a diverse relationship between serum Vit D levels and intensity of primary dysmenorrhea. This means that the severity of primary dysmenorrhea increases with decreasing serum Vit D levels. Clinical trial studies [28,31-35] have also shown that Vit D intake (in pill or capsule form, in high doses or other doses) reduces the severity of primary dysmenorrhea. In a study [29] comparing the effects of Vit D, Vit E, and ginger, all 3 factors contributed to reducing the severity of primary dysmenorrhea, with the effect being higher in the ginger group. Another study [30] comparing the effects of Vit D and Vit E on the severity of primary dysmenorrhea found that Vit D was more effective in reducing the severity of primary dysmenorrhea than Vit E. On comparing the effect of routine analgesics on reduced primary dysmenorrhea (nonsteroidal anti-inflammatory drug [NSAIDs]) alone or in combination with Vit D, a study [31] found that if Vit D was associated with analgesia, the rate of recovery was higher, such that many people in the Vit D and analgesics group used fewer analgesics at the end of the procedure. In one study [19], it was shown that calcium intake alone is more effective than calcium intake along with Vit D in reducing the severity of primary dysmenorrhea. In another study, 30 subjects who received calcium monotherapy and 30 subjects who received combined calcium and magnesium therapy had equal reduction in the severity of primary dysmenorrhea, but another study [29] found that omega-3 was more effective in reducing the severity of primary dysmenorrhea than calcium. The details of the studies are shown in Table 4.
In clinical trials (13 studies), the forms of Vit D used were drops or capsules of 50,000 units, 100 mg, 667 units, or 300,000 units. Calcium supplements were also available in the capsule form (alone or in combination with magnesium).
The duration of treatment for interventions ranged from 4 to 12 weeks, depending on their treatment protocol.
Most of the clinical trial studies had a placebo-controlled design. In one study, calcium was compared to fish oil. Vit D was also compared to Vit E, ginger, and analgesia.
In a study by Karacin et al. [18], serum calcium levels in the group of patients with primary dysmenorrhea were reported to be 8.3±0.7 mg/dL, which showed significant differences when compared with the control group. Among people with primary dysmenorrhea, 43.5% had hyperparathyroidism, which showed significant differences. Serum levels of alkaline phosphatase in the dysmenorrhea group were l80.4±24.8 IU, which showed insignificant differences.
In a study by Abdul-Razzak et al. [21], serum levels of calcium and alkaline phosphatase were normal in 82.1% and 80.4%, respectively. Furthermore, 48.2% of the study population had hyperparathyroidism.
In the study carried out by Kucukceran et al. [27], the serum calcium level was 9.9±0.3 in the insufficient group, 9.9±0.4 in the deficient group, and 9.5±0.5 mg/dL in the severely deficient group, which was not significant. Serum phosphorus levels were 3.4±0.5, 3.6±0.5 and 3.7±0.6 mg/ dL, respectively, which were not significant. Parathormone levels were 30.1±14, 45.6±17.6, and 46.1±15.8 picograms/ mL, respectively, which were significant.
In a study conducted by Moini et al. [2], serum calcium, phosphorus, and alkaline phosphatase levels at baseline in the Vit D group were reported to be 9.86±0.30 mg/dL, 3.50±0.27 mg/dL and 158.88±34.84 units/L, respectively, which were not significant.
Five studies [18,22,26,28,29] examined the effect of Vit D or calcium on associated systemic symptoms.
In the studies performed by Karacin et al. [18] and Abdul-Razzak et al. [21], it was found that people with abnormal Vit D levels were more likely to have systemic symptoms such as nausea, vomiting, headache, and fatigue; however, no relationship was observed with diarrhea. Early reports of depression, restlessness, mood swings, fatigue, headaches, breast tenderness, bloating, dizziness, diarrhea and nausea, and vomiting were reported. Bahrami et al. [28] found that intake of high doses of Vit D ameliorated back pain and crying easily at the end of the intervention. In the study by Mehrpooya et al. [31], the severity of nausea, vomiting, and breast tenderness in the omega-3 group was lower during the intervention; however, bloating was decreased more in the calcium group. Therefore, abnormal serum Vit D levels can play a role in causing or exacerbating systemic symptoms along with primary dysmenorrhea, and intake of Vit D and calcium supplements can improve symptoms.
Eventually, it can be concluded that low calcium levels increase uterine muscle contraction and can cause pain following decreased uterine blood flow. Low levels of Vit D can also increase primary dysmenorrhea by increasing prostaglandin production or decreasing calcium intestinal absorption. Vit D and calcium intake can be effective in reducing the severity of primary dysmenorrhea and reducing the rate of analgesic use.
In this systematic review, the results of high-quality studies on the role of Vit D and calcium in primary dysmenorrhea were analyzed. Overall, the results of the present study showed that low levels of Vit D were inversely related to the severity of primary dysmenorrhea and that Vit D and calcium intake could reduce the severity of primary dysmenorrhea.
According to the results of the present study, some studies did not report a significant relationship between serum calcium levels and the intensity of dysmenorrhea, while only one study reported a significant relationship. According to some studies, impaired calcium regulation has been shown to be a contributing factor in increasing the incidence and severity of menstrual pain [37], and even low plasma calcium levels in the premenstrual period have been reported in people with premenstrual syndrome (PMS), which may pertain to the role of calcium in controlling neurotransmitter activity. Thus, low calcium levels appear to increase spasm and contraction of the uterine muscles, which can cause pain following a decrease in uterine blood flow [38]. Therefore, given the contradictions in the results of studies, it seems that more research is quintessential in this field.
According to the present study, 2 studies did not show a relationship between serum Vit D levels and the severity of primary dysmenorrhea, whereas 2 research studies reported that there was an inverse relationship between serum Vit D levels and severity of primary dysmenorrhea. Recent studies have shown that Vit D deficiency is more common in patients with dysmenorrhea [26,37].
Vit D plays a central role in calcium homeostasis and is metabolized in 2 stages of hydroxylation [39]. However, Vit D plays a vital role in the female reproductive system. Vit D receptors have been found in ovarian and endometrial tissue, and in epithelial cells of the fallopian tubes, decidua, and placenta [40]. Vit D has also been reported to reduce the production of prostaglandins. Many studies have reported a close relationship between Vit D deficiency and early dysmenorrhea due to the regulatory action of calciferol on prostaglandin levels [41]. Studies have shown that metabolism and absorption of vitamins and minerals may play a major role in the development and treatment of menstrual disorders [42]. Two recent studies have reported an association between low calcium intake or Vit D deficiency and dysmenorrhea in adolescent and young females [26,40].
According to the results of modern studies, taking calcium supplements alone is more effective than taking it along with Vit D. Nutrition is one of the most important factors affecting quality of life. Metabolic and nutritional conditions may play an important role in the etiology and treatment of menstrual disorders, and menstrual pain is relieved with good nutrition. It has also been reported that there is a close relationship between calcium intake in food and reduced severity of primary dysmenorrhea [38]. The benefits of calcium supplements have been shown to reduce the severity of menstrual symptoms such as abdominal cramps, general pain, and back pain in some studies [43]. In another study, consuming 1,200 mg/d of calcium carbonate for 3 cycles reduced menstrual symptoms such as back pain and abdominal cramps [44]. Some studies also suggest that increasing the dose of calcium reduces the severity of dysmenorrhea such that none of the individuals with intake of 4 capsules per day complained of severe dysmenorrhea. This seems to pertain to the physiological function of calcium in controlling the contraction and tone of the uterine muscles [45], such that low calcium absorption through food increases uterine cramps and pain in women with primary dysmenorrhea [21]. The results of the present study showed that taking Vit D supplementation in any form or dose can be effective in reducing the severity of primary dysmenorrhea. High doses of Vit D3 (50,000 IU/ weekly) are recommended to prevent and treat Vit D deficiency. In addition, high Vit D intake may reduce the risk of PMS, possibly by affecting calcium levels, fluctuations in sex steroid hormones, or neurotransmitter function [46,47]. In a number of studies, Vit D fluctuations in the menstrual cycle have been reported with changes in estradiol during ovulation and during the luteal phase [37]. According to results of studies in Iran and Italy, taking a single oral dose of 300,000 units of cholecalciferol, 5 days before the onset of menstrual bleeding, reduces pain in severe primary dysmenorrhea [2]. Vit D appears to act in several ways to relieve endometrial pain in dysmenorrhea. In the endometrium, the expression of cyclooxygenase-2 decreases, and subsequently the production of prostaglandins also decreases [48]. Vit D may also act as an anti-inflammatory factor [49,50].
Studies have shown that serum levels of alkaline phosphatase are not related to the severity of primary dysmenorrhea, whereas there is a significant relationship between increased parathormone levels and severity of dysmenorrhea. Some studies have suggested a physiological effect of calcium on muscle contraction and expansion [47,48]. Since calcium homeostasis is mediated by the functions of calcitonin, parathyroid hormone, and 25-hydroxy Vit D3, it can be expected that these 3 hormones may play a role in the pathophysiology of primary dysmenorrhea [51]. When serum Vit D levels are reduced, intestinal calcium absorption is significantly reduced. Thereafter, the calcium in the extracellular fluid decreases and the secretion of parathyroid hormone increases. In turn, the parathyroid hormone increases renal reabsorption of calcium and intestinal absorption of calcium and phosphate [48], and hence, it appears that elevated parathyroid hormone levels may affect the severity of dysmenorrhea.
Among the articles under review, only 2 studies examined the relationship between phosphorus and primary dysmenorrhea, and did not report any significant statistical relationship between the 2. No specific studies have been performed in this field and only some articles have mentioned it as a secondary finding. More research seems to be needed in this area.
On a brief look at the relationship between serum Vit D or calcium levels and the severity of systemic symptoms along with early dysmenorrhea, among the articles studied, this relationship is significant and it seems that taking Vit D and calcium supplements can reduce the severity of these symptoms. Several studies have reported an inverse relationship between serum Vit D levels and the risk of depression, fibromyalgia, dysmenorrhea, and uterine fibroids [50,52]. Rahnemaie et al. [22] reported that serum Vit D levels were inversely related to the severity of fatigue, nausea and vomiting, and headache but not to the severity of diarrhea.
In one study, calcium was compared to fish oil. There is a dearth of evidence on the effect of fish oil on primary dysmenorrhea [53]. According to previous studies, fish oil consumption can produce prostacyclin and reduce the severity of primary dysmenorrhea. The effect of omega-3 fatty acid on dysmenorrhea may be due to the lower production of prostaglandins and leukotrienes [54]. Sadeghi et al. [55] showed that a combination of Vit E and omega-3 clearly reduced the severity of dysmenorrhea.
A study comparing the effects of Vit D, Vit E, and ginger showed that all 3 are effective in reducing the severity of primary dysmenorrhea, with more reduction in the ginger group. Ginger has long been used to reduce dysmenorrhea [56]. The mechanism for reducing pain by ginger is not fully understood. Evidence suggests that ginger contains substances such as gingerol and gingerdione that can act as anti-inflammatory and analgesics [57]. In addition, in vitro studies showed that ginger inhibits cyclooxygenase, followed by the production of prostaglandins and leukotrienes, and decreases the severity of dysmenorrhea [20]. It seems that Vit E can also reduce the severity of dysmenorrhea by increasing beta-endorphins [55]. The results revealed that ginger was more effective than Vit D and Vit E. However, further research is needed to compare the effect of routine analgesics on reducing the severity of primary dysmenorrhea (NSAIDs) alone or in combination with Vit D, which have been shown to be associated with Vit D analysis. The rate of relief was higher, with many people taking Vit D and analgesics using fewer analgesics at the end of the procedure. Given the limitations of comparative studies, further studies are needed in this regard [58].
Low levels of Vit D and calcium are inversely related to the severity of primary dysmenorrhea, and taking Vit D and calcium can reduce the severity of primary dysmenorrhea and its associated systemic symptoms. Therefore, the use of calcium and Vit D supplements can be recommended to relieve dysmenorrhea. One of the limitations of the present study was the small number of studies to investigate the effect of Vit D and calcium on the relief of dysmenorrhea. Therefore, further studies are warranted in this area.
Acknowledgments
We appreciate the Non-communicable Diseases Research Center, Alborz University of Medical Sciences.
Table 1
Search strategy
Table 2
Population, Intervention, Comparators, Outcomes, and Study Design (PICOS) criteria for this study
Table 3
Risk of bias summary: authors’ judgements about each risk of bias item for each included study
| Reference | Domains | ||||
|---|---|---|---|---|---|
| Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | |
| Pakniat et al. [20] | + | + | + | + | + |
| Ayşegül et al. [29] | + | + | + | + | + |
| Lama et al. [30] | + | + | + | + | + |
| Kucukceran et al. [27] | − | + | − | + | + |
| Bahrami et al. [28] | − | ? | − | + | + |
| Zarei et al. [19] | + | + | + | + | + |
| Mehrpooya et al. [31] | + | + | + | + | + |
| Charandabi et al. [32] | + | + | + | + | + |
| Fareena Begum et al. [33] | + | + | + | + | + |
| Moini et al. [2] | + | + | + | + | + |
| Ataee et al. [34] | + | + | + | + | + |
| Zangene et al. [35] | + | + | + | + | + |
| Lasco et al. [36] | + | + | + | + | + |
Table 4
Characteristics of the studies included in the systematic review
| Author | Region | Design | Sample size | Age (yr) | Menarche age (yr) | BMI (kg/m2) | Duration of each menstrual dysmenorrhea (day) | Duration of menstruation cycle (day) | Intervention | Control | Pain intensity | Serum level of Vit D | Assessment tools | Definition | Results | Quality | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||||
| Baseline | After | Baseline | After | |||||||||||||||
| Rahnemaie et al. [22] | Iran | Cross sectional | 143 | 22.52±2.97 | 13.46±1.03 | 23.53±3.70 | 2.43±1.32 | NR | - | - | 6.91±1.59 | - | 24.45±11.85 ng/mL | - | VAS | ≥4 | Not significant | 21a) |
|
|
||||||||||||||||||
| Zeynali et al. [25] | Iran | Cross sectional | 372 | 22.40±2.01 | 13.21±1.44 | 24.03±3.07 | 2.18±1.04 | 6.09±1.11 | - | - | - | - | VAS | ≥1 | Significant | 20a) | ||
|
|
||||||||||||||||||
| Karacin et al. [18] | Turkey | Case control | 368 | 20.80 | 12.15 | 22.26 | NR | 5.1 | - | - | 7.30±1.40 | - | 7.10±3.80 ng/mL | - | VAS | 1-10 | Significant | 20a) |
|
|
||||||||||||||||||
| Abdul- Razzak et al. [26] | Jordan | Cross sectional | 56 | 21.90±2.76 | 13.60±1.40 | NR | NR | NR | - | - | - | - | NRS | 0-10 | Not significant | 19a) | ||
|
|
||||||||||||||||||
| Kucukceran et al. [27] | Turkey | Quasi experiment | 100 | 20.50 | 13.13 | 21.42 | 2.33 | 5.86 | 7.00±2.00 | 4.10±1.60 | 13.90±6.10 | 31.10±3.90 | VAS | 0-10 | Significant | 18b) | ||
|
Duration: 2 mon Maintenance therapy: (6 drops of Vit D3 per day) for 1 mon |
||||||||||||||||||
|
|
||||||||||||||||||
| Bahrami et al. [28] | Iran | Quasi experiment | 897 | 14.72±1.50 | 12.57±1.19 | NR | NR | 71.8% normal cycle | High-dose Vit D supplements (as 50,000 IU/wk of cholecalciferol) | - | 22.70±22.60 nmol/mL | 89.90±38.30 nmol/mL | VIPS | 0-5 | Significant | 18b) | ||
| Duration: 1 capsule over 9 wk | ||||||||||||||||||
|
|
||||||||||||||||||
| Pakniat et al. [20] | Iran | RCT | 200 | 22.44±1.92 | 12.55±1.00 | 21.62±3.15 | 2.60±0.89 | 5.20±0.95 | Placebo+ mefenamic acid 250 mg | 7.13±0.80 | 4.93±1.48 | - | - | VAS | 0-10 | Significant | 22b) | |
|
Drop on: 2 day before the onset of menstrual flow Drop out: 3 day after the onset of menstrual flow Duration: 5 day in a cycle |
||||||||||||||||||
|
|
||||||||||||||||||
| Ayşegül et al. [29] | Turkey | RCT | 142 | 22.00 | 23.40±5.60 | NR | NR | NR | 8.50±1.20 | 4.90±2.40 | - | - | VAS | 1-10 | Vit D more significant | 20b) | ||
|
Drop on: 2 days before the expected date of menstruation Drop out: first 3 day of menstruation Duration: 5 day in 2 consecutive cycles |
||||||||||||||||||
|
|
||||||||||||||||||
| Lama et al. [30] | Saudi Arabia | RCT | 22 | 13-40 | NR | NR | NR | NR |
50,000 IU Vit D/wk+their usual analgesics regimen Duration: 8 wk |
Their usual analgesics regimen | 7.80 | 3.60 | 30.10±13.40 nmol/L | 80.20±14.30 nmol/L | VAS | 0-10 | Significant | 20b) |
|
|
||||||||||||||||||
| Zarei et al. [19] | Iran | RCT | 85 | 23.66 | 13.10 | 21.83 | NR | NR | Placebo | NR | NR | VAS | 0-10 | Significant for calcium | 22b) | |||
|
Drop on: from 15th cycle day Drop out: until menstrual pain disappearance Duration: 3 cycles |
||||||||||||||||||
|
|
||||||||||||||||||
| Mehrpooya et al. [31] | Iran | RCT | 80 | 25.24 | NR | 23.16 | NR | 5.80 | - | - | VAS | ≥4 | Significant | 20b) | ||||
|
Drop on: every day in the first cycle and 8 day of second and third cycle Drop out: till 2 day after initiation of menstruation Duration: 3 cycles |
||||||||||||||||||
|
|
||||||||||||||||||
| Charandabi et al. [32] | Iran | RCT | 61 | 21.00±2.20 | 12.60 | 22.30±3.00 | NR | 6.2 | Placebo | - | - | VAS | ≥5 | Significant | 20b) | |||
|
Drop on: 1 pill a day, from the day 15th of their cycle Drop out: till the day with no menstrual pain Duration: 2 cycles |
||||||||||||||||||
|
|
||||||||||||||||||
| Fareena Begum et al. [33] | India | RCT | 50 | 21.18 | 62% earlier onset of menarche | NR | NR | Placebo | 8.76±0.97 | 3.56±0.76 | 17.84±10.1 ng/mL | 34.70±8.10 ng/mL | VAS | 0-10 | Significant | 22b) | ||
| Followed up: for the next 2 and 4 mon | ||||||||||||||||||
|
|
||||||||||||||||||
| Moini et al. [2] | Iran | RCT | 50 | 26.36 | 12.72 | 22.95 | NR | NR |
50,000 IU oral vit D/wk Duration: 8 wk |
Placebo | 7.80 | 2.80 | 9.69±5.09 ng/mL | 55.44±6.02 ng/mL | VAS | 0-10 | Significant | 22b) |
|
|
||||||||||||||||||
| Ataee et al. [34] | Iran | RCT | 54 | NR | NR | NR | NR | NR | Placebo | 7.53±1.85 | 3.77±1.77 | 7.28±3.64 ng/mL | - | VAS | 0-10 | Significant | 22b) | |
|
Drop on: 5 day before the beginning of menstruation Drop out: beginning of menstruation Duration: 2 cycles |
||||||||||||||||||
|
|
||||||||||||||||||
| Zangene et al. [35] | Iran | RCT | 54 | 22.43 | NR | 21.03 | NR | NR | Placebo | 7.53±1.85 | 3.77±1.78 | 7.37 ng/mL | NR | VAS | 0-10 | Significant | 20b) | |
|
Drop on: 5 day before menstruation Drop out: onset of menstruation Duration: 3 cycles |
||||||||||||||||||
|
|
||||||||||||||||||
| Lasco et al. [36] | Italy | RCT | 40 | 26.65 | NR | 21.56 | NR | NR | Single oral dose of cholecalciferol (300,000 IU/1 mL | Placebo | 5.85±2.00 | 3.50±1.27 | 27.19±7.53 ng/mL | - | VAS | 0-10 | Significant | 22b) |
|
Drop on: 5 day before the putative beginning of their next menstrual cycle Drop out: onset of menstruation Duration: 2 mon |
||||||||||||||||||
References
1. Bajalan Z, Alimoradi Z, Moafi F. Nutrition as a potential factor of primary dysmenorrhea: a systematic review of observational studies. Gynecol Obstet Invest 2019;84:209-24.


2. Moini A, Ebrahimi T, Shirzad N, Hosseini R, Radfar M, Bandarian F, et al. The effect of vitamin D on primary dysmenorrhea with vitamin D deficiency: a randomized double-blind controlled clinical trial. Gynecol Endocrinol 2016;32:502-5.


3. Firouzi M, Zahedifard T, Salari P, Mazlom S. Comparing the pattern of primary dysmenorrhea before and after childbirth. J Midwifery Reprod Health 2019;7:1514-21.
4. Azagew AW, Kassie DG, Walle TA. Prevalence of primary dysmenorrhea, its intensity, impact and associated factors among female students’ at Gondar town preparatory school, Northwest Ethiopia. BMC Womens Health 2020;20:5.



5. Monday I, Anthony P, Olunu E, Otohinoyi D, Abiodun S, Owolabi A, et al. Prevalence and correlation between diet and dysmenorrhea among high-school and college students in Saint Vincent and Grenadines. Open Access Maced J Med Sci 2019;7:920-4.




6. Chen L, Tang L, Guo S, Kaminga AC, Xu H. Primary dysmenorrhea and self-care strategies among Chinese college girls: a cross-sectional study. BMJ Open 2019;9:e026813.



7. Vlachou E, Owens DA, Lavdaniti M, Kalemikerakis J, Evagelou E, Margari N, et al. Prevalence, wellbeing, and symptoms of dysmenorrhea among university nursing students in Greece. Diseases 2019;7:1-14.

8. Femi-Agboola DM, Sekoni OO, Goodman OO. Dysmenorrhea and its effects on school absenteeism and school activities among adolescents in selected secondary schools in Ibadan, Nigeria. Niger Med J 2017;58:143-8.



9. Acheampong K, Baffour-Awuah D, Ganu D, Appiah S, Pan X, Kaminga A, et al. Prevalence and predictors of dysmenorrhea, its effect, and coping mechanisms among adolescents in Shai Osudoku District, Ghana. Obstet Gynecol Int 2019 2019:5834159.

10. Kazama M, Maruyama K, Nakamura K. Prevalence of dysmenorrhea and its correlating lifestyle factors in Japanese female junior high school students. Tohoku J Exp Med 2015;236:107-13.


11. Al-Matouq S, Al-Mutairi H, Al-Mutairi O, Abdulaziz F, Al-Basri D, Al-Enzi M, et al. Dysmenorrhea among high-school students and its associated factors in Kuwait. BMC Pediatr 2019;19:80.



12. Sidi I, Hounkpatin B, Obossou AAA, Salifou K, Vodouhe M, Denakpo J, et al. Primary dysmenorrhea in the schools of Parakou: prevalence, impact and therapeutic approach. Gynecol Obstet (Sunnyvale) 2016;6:376.

13. Aktaş D. Prevalence and factors affecting dysmenorrhea in female university students: effect on general comfort level. Pain Manag Nurs 2015;16:534-43.


14. Arafa AE, Senosy SA, Helmy HK, Mohamed AA. Prevalence and patterns of dysmenorrhea and premenstrual syndrome among Egyptian girls (12-25 years). Middle East Fertil Soc J 2018;23:486-90.

15. Habibi N, Huang MS, Gan WY, Zulida R, Safavi SM. Prevalence of primary dysmenorrhea and factors with its intensity among undergraduate students: a cross sectional study. Pain Manag Nurs 2015;16:855-61.


16. Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update 2015;21:762-78.


17. Salehi A, Marzban M. Effect of foeniculum vulgare on primary dysmenorrhea: a systematic review and meta-analysis. Womens Health Bull Forthcoming 2018.
18. Karacin O, Mutlu I, Kose M, Celik F, Kanat-Pektas M, Yilmazer M. Serum vitamin D concentrations in young Turkish women with primary dysmenorrhea: a randomized controlled study. Taiwan J Obstet Gynecol 2018;57:58-63.


19. Zarei S, Mohammad-Alizadeh-Charandabi S, Mirghafourvand M, Javadzadeh Y, Effati-Daryani F. Effects of calcium-vitamin d and calcium-alone on pain intensity and menstrual blood loss in women with primary dysmenorrhea: a randomized controlled trial. Pain Med 2017;18:3-13.


20. Pakniat H, Chegini V, Ranjkesh F, Hosseini MA. Comparison of the effect of vitamin E, vitamin D and ginger on the severity of primary dysmenorrhea: a single-blind clinical trial. Obstet Gynecol Sci 2019;62:462-8.



21. Abdul-Razzak KK, Ayoub NM, Abu-Taleb AA, Obeidat BA. Influence of dietary intake of dairy products on dysmenorrhea. J Obstet Gynaecol Res 2010;36:377-83.


22. Rahnemaie FS, Afrakhteh M, Nasiri M, Zayeri F, Vafa M, Ozgoli G. Relationship between serum vitamin D with severity of primary dysmenorrhea and associated systemic symptoms in dormitories students of Shahid Beheshti University of Medical Sciences. Iran J Obstet Gynecol Infertil 2019;22:44-53.
23. Abdi F, Ozgoli G, Rahnemaie FS. A systematic review of the role of vitamin D and calcium in premenstrual syndrome. Obstet Gynecol Sci 2019;62:73-86.



24. Rahnemaie FS, Zare E, Zaheri F, Abdi F. Effects of complementary medicine on successful breastfeeding and its associated issues in the postpartum period. Iran J Pediatr 2019;29:e80180.

25. Zeynali M, Haghighian HK. Is there a relationship between serum vitamin D with dysmenorrhea pain in young women? J Gynecol Obstet Hum Reprod 2019;48:711-4.


26. Abdul-Razzak KK, Obeidat BA, Al-Farras MI, Dauod AS. Vitamin D and PTH status among adolescent and young females with severe dysmenorrhea. J Pediatr Adolesc Gynecol 2014;27:78-82.


27. Kucukceran H, Ozdemir O, Kiral S, Berker DS, Kahveci R, Ozkara A, et al. The impact of circulating 25-hydroxyvitamin and oral cholecalciferol treatment on menstrual pain in dysmenorrheic patients. Gynecol Endocrinol 2019;35:53-7.


28. Bahrami A, Avan A, Sadeghnia HR, Esmaeili H, Tayefi M, Ghasemi F, et al. High dose vitamin D supplementation can improve menstrual problems, dysmenorrhea, and premenstrual syndrome in adolescents. Gynecol Endocrinol 2018;34:659-63.


29. Ayşegül Ö, Seda A, Şevket O, Özdemir M, İlhan G, Davutoğlu E. A randomized controlled study of vitamin D in the treatment of primary dysmenorrhea. Duzce Med J 2019;21:32-6.

30. Lama A, Najla A, Azah A, Areej A, Alaa E, Salem A. Vitamin D supplements as adjunctive therapy with analgesics for primary dysmenorrhea: a randomized clinical trial. Int J Reprod Med Gynecol 2019;5:004-14.
31. Mehrpooya M, Eshraghi A, Rabiee S, Larki-Harchegani A, Ataei S. Comparison the effect of fish-oil and calcium supplementation on treatment of primary dysmenorrhea. Rev Recent Clin Trials 2017;12:148-53.


32. Charandabi SM, Mirghafourvand M, Chegini S, Javadzadeh Y. Calcium with and without magnesium for primary dysmenorrhea: a double-blind randomized placebo controlled trial. Int J Women Health Reprod Sci 2017;5:332-8.

33. Fareena Begum A. Study of prevalence of vitamin D deficiency in primary dysmenorrhea and administration of a single oral dose of vitamin D to improve primary dysmenorrhea. Coimbatore: Coimbatore Medical College; 2017.
34. Ataee M, Zangeneh M, Mahboubi M. Cholecalciferol for Primary Dysmenorrhea in a College aged Population—A Clinical Trial. J Biol Todays World 2015;4:54-7.

35. Zangene M, Veisi F, Nankali A, Rezaei M, Ataee M. Evaluation of the effects of oral vitamin-d for pelvic pain reduction in primary dysmenorrhea. Iran J Obstet Gynecol Infertil 2014;16:14-20.

36. Lasco A, Catalano A, Benvenga S. Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: results of a randomized, double-blind, placebo-controlled study. Arch Intern Med 2012;172:366-7.


37. Thys-Jacobs S. Micronutrients and the premenstrual syndrome: the case for calcium. J Am Coll Nutr 2000;19:220-7.


38. Thys-Jacobs S, McMahon D, Bilezikian JP. Cyclical changes in calcium metabolism across the menstrual cycle in women with premenstrual dysphoric disorder. J Clin Endocrinol Metab 2007;92:2952-9.


39. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004;80:1689S-1696S.


40. Anagnostis P, Karras S, Goulis DG. Vitamin D in human reproduction: a narrative review. Int J Clin Pract 2013;67:225-35.


41. Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res 2005;65:7917-25.


42. Proctor ML, Murphy PA. Herbal and dietary therapies for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev 2001;CD002124.


43. Das B, Prasanna Chandra M, Samanta S, Mallick AK, Sowmya MK. Serum inorganic phosphorus, uric acid, calcium, magnesium and sodium status during uterine changes of menstrual cycle. Int J Biomed Res 2012;3:209-13.

44. Thys-Jacobs S, Starkey P, Bernstein D, Tian J. Premenstrual Syndrome Study Group. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual symptoms. Am J Obstet Gynecol 1998;179:444-52.


45. Marshall WJ, Bangert SK. Clinical chemistry. Edinburgh: Mosby; 2008.
46. Bertone-Johnson ER. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev 2009;67:481-92.



48. Hashemipour S, Larijani B, Adibi H, Javadi E, Sedaghat M, Pajouhi M, et al. Vitamin D deficiency and causative factors in the population of Tehran. BMC Public Health 2004;4:38.




49. Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol 2011;51:311-36.


50. Bertone-Johnson ER, Manson JE. Vitamin d for menstrual and pain-related disorders in women: comment on “improvement of primary dysmenorrhea caused by a single oral dose of vitamin D”. Arch Intern Med 2012;172:367-9.


51. Maïmoun L, Sultan C. Effect of physical activity on calcium homeostasis and calciotropic hormones: a review. Calcif Tissue Int 2009;85:277-86.


52. Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin D and the risk of uterine fibroids. Epidemiology 2013;24:447-53.



54. Zamani M, Arab M, Nasrollahi S, Manikashani K. The evaluation of fish oil (Omega-3 fatty acids) efficacy in treatment of primary dysmenorrhea in high school female students in Hamadan. J Gorgan Univ Med Sci 2005;7:39-42.
55. Sadeghi N, Paknezhad F, Rashidi Nooshabadi M, Kavianpour M, Jafari Rad S, Khadem Haghighian H. Vitamin E and fish oil, separately or in combination, on treatment of primary dysmenorrhea: a double-blind, randomized clinical trial. Gynecol Endocrinol 2018;34:804-8.


56. Daily JW, Zhang X, Kim DS, Park S. Efficacy of ginger for alleviating the symptoms of primary dysmenorrhea: a systematic review and meta-analysis of randomized clinical trials. Pain Med 2015;16:2243-55.


- TOOLS
-
METRICS

- Related articles in Obstet Gynecol Sci
-
Role of microRNAs as biomarkers of cervical carcinogenesis: a systematic review2021 September;64(5)
A systematic review of the role of vitamin D and calcium in premenstrual syndrome2019 March;62(2)
The effect of bee prepolis on primary dysmenorrhea: a randomized clinical trial2019 September;62(5)