|
 |
- Search
| Obstet Gynecol Sci > Volume 59(6); 2016 > Article |
This article has been corrected. See Obstet Gynecol Sci. 60(3): 322.
Abstract
Objective
To evaluate the effect of orally administered dienogest (DNG) for dysmenorrhea and pelvic pain associated with endometriosis.
Methods
For this study we recruited 89 patients with dysmenorrhea and pelvic pain associated with endometriosis diagnosed by laparoscopy. All patients complained of persistent dysmenorrhea and pelvic pain despite surgical treatment 6 months previously. After 6 months of DNG treatment, we used a 0 to 3 point verbal rating scale to measure the severity of disability in daily life due to dysmenorrhea and pelvic pain, and the use of analgesics. Weight gain, serum lipid and liver enzyme tests were performed before treatment and after 6 months of DNG treatment.
Results
Total dysmenorrhea scores assessed by the verbal rating scale significantly decreased by the end of treatment (P<0.001). The mean (±standard deviation) pain score for dysmenorrhea before and after treatment were 1.42±1.1 and 0.1±0.3, respectively. The mean non-menstrual pelvic pain scores before and after treatment were 0.52±0.6 and 0.18±0.3, respectively, showing a significant difference (P<0.001). The use of analgesics significantly decreased by the end of the treatment (P<0.001). The associated adverse effects were weight gains (in 56 of 89 patients, 63%) and uterine bleeding (in 28 of 89 patients, 31.5%). The weight gain (before treatment, 57.9±9.7; after treatment, 61.1±12.6) was statistically significant (P<0.040).
Dysmenorrhea is the most common symptom in patients with endometriosis [1]. Endometriosis is defined as the presence of endometrial glands and stroma outside the uterus. It is a chronic gynecologic disease. Symptoms include chronic pelvic pain, dysmenorrhea, dyspareunia, and infertility in reproductive-age women.
Current guidelines for the medical treatment of endometriosis recommend hormonal therapies that suppress ovarian function to decrease the serum estradiol concentration and thus shrink the lesions. Gonadotropin-releasing hormone agonists (GnRH-a) are effective. Danazol, progestin, and estrogen/progestin combinations are also used [2].
Although a GnRH-a exhibits considerable efficacy by decreasing the serum estradiol concentration to postmenopausal levels, such agents are accompanied by a high incidence of hypoestrogenic symptoms, and their long-term use is associated with a substantial decrease in bone mineral density, limiting the duration of use [3].
There is, therefore, a need for new and highly effective drugs that can be used over a longer term. The progestin dienogest (DNG) was developed for oral contraception, endometriosis treatment, and menopause management. DNG is a synthetic progestogen that acts as a specific progesterone receptor agonist with anti-androgenic activity and strong oral endometrial activity [4].
DNG (Visanne, Bayer HealthCare, Berlin, Germany) is a selective progestin that has recently been approved for the treatment of endometriosis in Europe, Japan, and other countries, at a low oral dose of 2 mg/day. DNG reduces endometriosis lesions by creating a local progestogenic environment, while only moderately suppressing systemic estrogen levels [5,6].
Therefore, we performed a clinical trial to examine the effect of oral administration of 2 mg DNG on dysmenorrhea associated with endometriosis. The aim of this study was to determine the effects of DNG, and compare the results with previous reports.
The study was conducted at the Department of Obstetrics and Gynecology at Chosun University Hospital. Between July 2013 and July 2015, 105 reproductive-age women (range, 22 to 53 years) who had endometriosis were enrolled in this non-randomized prospective study. Of 16 patients who did not completed the study, 9 were lost to follow up, 6 had inadequate charting, and 1 was excluded because of severe bleeding. Inclusion criteria included histologically-proven endometriosis (stages I to IV, based on revised American Society of Reproductive Medicine scoring) [7]. Exclusion criteria were amenorrhea of 3 or more months, a need for primary surgical treatment of endometriosis, use of hormonal agents within 1 to 6 months of screening (depending on the class of agent) or abnormal findings on gynecological examination other than endometriosis. The institutional review board approved the study, and informed consent was obtained from all women.
Of those evaluated for DNG use were, 69 patients (78%) had recurrent pain despite 6 months of GnRH-a use after surgery, 20 (22%) with recurrent pain had been diagnosed endometriosis prior to surgery. Treatment with DNG began on the 3rd day (±2 days) of the menstrual cycle and continued for 6 cycles. During the 2 menstrual cycles before starting the study, each patient underwent a pre-recruitment evaluation, consisting of a general medical and gynecologic history, a physical and pelvic examination, a clinical evaluation of the signs and symptoms, a patient evaluation of pain, and a review of the menstrual record, including scoring of dysmenorrhea and non-menstrual pain, and recording of details about the use of analgesics. The effect of treatment was evaluated at the final visit after completing the treatment course.
Patients included in the study had dysmenorrhea or pelvic pain scoring higher than 3 points at the admission visit by using a modified pain scale originally developed by Biberoglu and Behrman [8] and Andersch and Milsom [9]. This verbal rating scale (VRS) defines pain according to limitations on the ability to work and the need for analgesics, based on the number of days analgesics are used.
The severity of dysmenorrhea and non-menstrual pelvic pain was scored by using a scale ranging from 0 to 3 (0=no pain, 1=some loss of work (or study) efficiency, 2=need for some rest, with loss of work, and 3=rest for more than 1 day); the use of analgesics was similarly scored (0=none, 1=analgesic use for 1 day, 2=analgesic use for 2 days, and 3=analgesic use for >3 days).
Body weight was measured 6 months after treatment ended with DNG. Serum lipids and liver enzyme tests were performed before, and after 6 months of treatment with DNG. The adverse effects were investigated by questionnaire after the treatment ended. Weight gain was defined as an increase of over 1 kg, and uterine bleeding as bleeding more than 10 days.
A paired t-test was used to compare changes in dysmenorrhea, and non-menstrual pelvic pain scores, use of analgesics, weight, serum lipid, and liver enzyme levels before and after treatment with DNG. A P-value of <0.05 indicated statistical significance. Statistical analysis was performed with SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
The demographic data are summarized in Table 1. Patients had mean body weight of 57.9±9.7 kg, mean height of 159.5±5.6 cm, and a body mass index of 22.8±3.6 kg/m2. All patients had endometriosis diagnosed by laparoscopy. All patients complained of persistent dysmenorrhea and pelvic pain, despite surgical treatment 6 months previously. A total of 77 patients (86%) were classified as having stage III (moderate) or higher endometriosis, and 12 (14%) as having stage I-II. However, the pain and staging were not correlated.
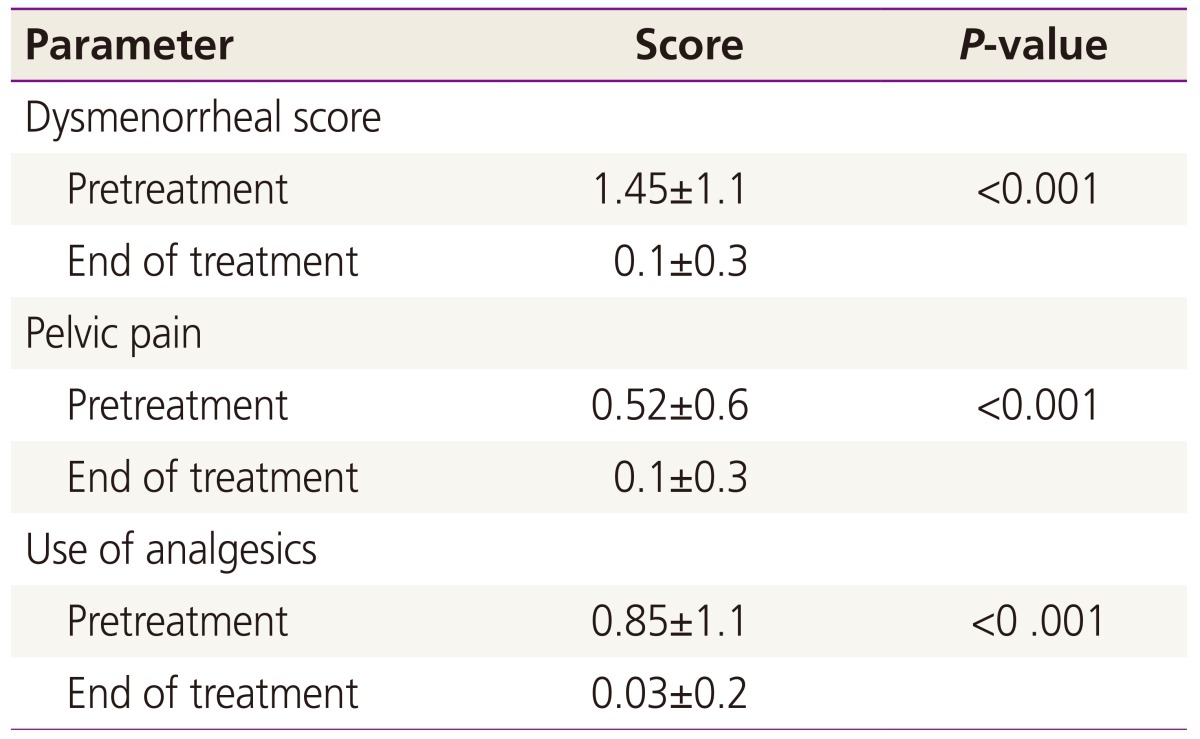
We determined the dysmenorrhea, and non-menstrual pelvic pain scores, and use of analgesics in 89 patients before and after treatment with DNG (Table 2). The effective endpoint was the patient response to treatment for dysmenorrhea and pelvic pain associated with endometriosis, as evaluated by the VRS. The analysis assessed changes in symptom severity before and after treatment with DNG. The mean (±standard deviation) score for dysmenorrhea was 1.42±1.1 before treatment and 0.1±0.3 after treatment. The dysmenorrhea score significantly decreased by the end of treatment (P<0.001). The mean non-menstrual pelvic pain score was 0.52±0.6 before treatment and 0.18±0.3 after treatment, a significant difference (P<0.001). The use of analgesics decreased significantly by the end of treatment (P<0.001).
There were a few side effects in this study (Table 3). Irregular uterine bleeding occurred in 28 of 89 cases (31.5%); however, only 1 patient discontinued treatment because of severe uterine bleeding. Moreover, irregular uterine bleeding decreased with continued treatment and resolved by the end of treatment. Another adverse effect was weight gain, a common adverse effect with other progestins used in endometriosis treatment. Weight after treatment (61.1±12.6 kg) was greater than that before treatment (57.9±9.7 kg); the increase was statistically significant (P<0.040). A weight gain of 15 kg was recorded in 1 patient. Other than uterine bleeding and weight gain, no serious adverse events related to DNG use occurred. Laboratory safety parameters showed no clinically relevant changes in serum lipid and liver enzyme levels with DNG treatment.
Some patients with endometriosis are asymptomatic. However, most have symptoms different intensity. The major symptoms are dysmenorrhea, chronic pelvic pain, and deep dyspareunia [10]. This study demonstrated that DNG was effective in decreasing endometriosis symptom scores. Several progestins and synthetic steroids such as drospirenone, trimegestone, nestorone, nomegestrol acetate, and DNG, which mimic the action of endogenous progesterone, have been developed for use as contraceptives and in hormone replacement therapy, and for treatment of gynecological disease, such as endometriosis [11]. DNG was approved for the treatment of endometriosis in the EU (as Visanne) in December 2009. It was also approved in Japan (as Dinagest 1 mg) in October 2007. Harada et al. [12] demonstrated that DNG is as effective as a GnRH-a for relieving the pain associated with endometriosis, but with a significantly lower decrease in bone mineral density and fewer hot flushes, which are typical side-effects of a GnRH-a. However, it is known that irregular genital bleeding occurs more frequently with DNG than with a GnRH-a.
Although laparoscopic diagnosis and treatment for endometriosis are currently used worldwide, Alkatout et al. [13] showed that combined surgical and hormone therapy (3.75 mg GnRH-a, monthly for 3 months) for endometriosis was the most effective treatment, especially for the reduction of symptoms (dysmenorrhea, pelvic pain, and others).
The present study also used combined surgical and hormone therapy, but with a difference. DNG, which may be used for 1 year or more [13,14], was used instead of a GnRH-a which has a limited duration of use, as it decreases bone mineral density. A comparative trial of DNG (2 mg/day) versus a GnRH-a [15,16] concluded that DNG is as effective for the relief of pain associated with endometriosis [15]. The proportion of women free of pelvic pain and dysmenorrhea at 24 weeks was approximately 60% in both treatment arms: 82% in the DNG group and 90% in the GnRH-a group, respectively [16].
Various adverse effects of hormone therapy for the treatment of endometriosis have been reported, including uterine bleeding, weight gain, headache, nausea, and hot flushes. In this study, patients complained of weight gain, uterine bleeding, fatigue, insomnia, breast tenderness, depression, dizziness, and other symptoms (Table 3). The most common adverse effects were uterine bleeding and weight gain.
Horie et al. [15] reported rates of genital bleeding in DNG and GnRH-a groups of 122 of 129 (95%) and 85 of 126 (67%), respectively. However, this adverse effect resolved by the end of treatment, as seen in our study. Several clinical trials have shown that contraceptive pills containing DNG provide good cycle control, with shorter and lighter bleeding than other contraceptives [17,18]. At the same DNG dose (2 mg/day), the choice of contraceptive can have a significant influence on the incidence rate of irregular vaginal bleeding [19].
The incidence rate of irregular bleeding in our study (28 of 89, 31.5%) was less than that (61.6%) reported for DNG in another study, in which the patients were prescribed DNG, 1 mg orally twice a day for 16 weeks [19]. The incidence of genital bleeding decreased with continued treatment and resolved by the end of treatment in our study. When uterine bleeding persisted for more than 10 days of DNG treatment, the medication was discontinued. After the uterine bleeding had completely dissolved DNG treatment was resumed, after which the cycle was maintained with no uterine bleeding.
The selective steroid receptor activities of DNG lead to fewer adverse effects, such as hirsutism, acne, weight gain, unfavorable carbohydrate and lipid metabolism compared to previous synthetic progestins, such as medroxyprogesterone acetate or norethisterone [6].
The data in the present study showed weight increase by the end of treatment. A previous paper reported that the absence of weight gain with DNG, but the present study showed a statistically significant difference (P<0.040).
Clinical evidence for the efficacy of DNG is lacking. Our results demonstrated that oral administration of 2 mg DNG effectively decreased dysmenorrhea associated with endometriosis. No serious adverse events were observed. Data from long-term studies indicated that pain relief continued to improve with sustained DNG therapy for 1 year and beyond [14,20]. DNG can also induce decidualization of ectopic endometrial tissue and atrophy of lesions with continued treatment by creating a hypoestrogenic and hyperprogestogenic endocrine environment; thus, DNG is effective in treating endometriosis at a dose of 2 mg daily [6]. Our results support the common clinical practice of treating dysmenorrhea resulting from endometriosis with oral administration of 2 mg DNG. This study demonstrated that DNG was effective in decreasing the scores of all endometriosis symptoms by the end of treatment.
In conclusion, the present study clearly demonstrated that orally administered DNG can be used to effectively treat dysmenorrhea and pelvic pain associated with endometriosis, although the side effects of weight gain and uterine bleeding should be considered. DNG is effective for pain control, the main focus of treatment for endometriosis. Despite its effectiveness, there were a few limitations. Previously unreported weight gain was statistically meaningful, but the effect of long-term treatment for more than 6 months is unknown.
References
1. Harada T, Momoeda M, Taketani Y, Hoshiai H, Terakawa N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril 2008;90:1583-1588. PMID: 18164001.


3. Crosignani P, Olive D, Bergqvist A, Luciano A. Advances in the management of endometriosis: an update for clinicians. Hum Reprod Update 2006;12:179-189. PMID: 16280355.


4. Sasagawa S, Shimizu Y, Kami H, Takeuchi T, Mita S, Imada K, et al. Dienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharmacokinetic profile. Steroids 2008;73:222-231. PMID: 18061638.


5. Oettel M, Breitbarth H, Elger W, Graser T, Hubler D, Kaufmann G, et al. The pharmacological profile of dienogest. Eur J Contracept Reprod Health Care 1999;4(Suppl 1):2-13.

6. McCormack PL. Dienogest: a review of its use in the treatment of endometriosis. Drugs 2010;70:2073-2088. PMID: 20964453.


7. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67:817-821. PMID: 9130884.


8. Biberoglu KO, Behrman SJ. Dosage aspects of danazol therapy in endometriosis: short-term and long-term effectiveness. Am J Obstet Gynecol 1981;139:645-654. PMID: 6452062.


9. Andersch B, Milsom I. An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol 1982;144:655-660. PMID: 7137249.


10. Petta CA, Matos AM, Bahamondes L, Faundes D. Current practice in the management of symptoms of endometriosis: a survey of Brazilian gynecologists. Rev Assoc Med Bras (1992) 2007;53:525-529. PMID: 18157368.


11. Sitruk-Ware R. New progestogens: a review of their effects in perimenopausal and postmenopausal women. Drugs Aging 2004;21:865-883. PMID: 15493951.


12. Harada T, Momoeda M, Taketani Y, Aso T, Fukunaga M, Hagino H, et al. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis: a randomized, double-blind, multicenter, controlled trial. Fertil Steril 2009;91:675-681. PMID: 18653184.


13. Alkatout I, Mettler L, Beteta C, Hedderich J, Jonat W, Schollmeyer T, et al. Combined surgical and hormone therapy for endometriosis is the most effective treatment: prospective, randomized, controlled trial. J Minim Invasive Gynecol 2013;20:473-481. PMID: 23567095.


14. Dallenbach-Hellweg G. The influence of contraceptive steroids on the histological appearance of the endometrium. In: Diczfalusy E, Fraser IS, Webb FT, editors. Endometrial bleeding and steroidal contraception. Bath: Pitman Press; 1980. p. 153-173.
15. Horie S, Harada T, Mitsunari M, Taniguchi F, Iwabe T, Terakawa N. Progesterone and progestational compounds attenuate tumor necrosis factor alpha-induced interleukin-8 production via nuclear factor kappa B inactivation in endometriotic stromal cells. Fertil Steril 2005;83:1530-1535. PMID: 15866594.


16. Strowitzki T, Marr J, Gerlinger C, Faustmann T, Seitz C. Detailed analysis of a randomized, multicenter, comparative trial of dienogest versus leuprolide acetate in endometriosis. Int J Gynaecol Obstet 2012;117:228-233. PMID: 22459918.


17. Zeun S, Lu M, Uddin A, Zeiler B, Morrison D, Blode H. Pharmacokinetics of an oral contraceptive containing oestradiol valerate and dienogest. Eur J Contracept Reprod Health Care 2009;14:221-232. PMID: 19565420.


18. Endrikat J, Parke S, Trummer D, Schmidt W, Duijkers I, Klipping C. Ovulation inhibition with four variations of a four-phasic estradiol valerate/dienogest combined oral contraceptive: results of two prospective, randomized, open-label studies. Contraception 2008;78:218-225. PMID: 18692612.


19. Cosson M, Querleu D, Donnez J, Madelenat P, Konincks P, Audebert A, et al. Dienogest is as effective as triptorelin in the treatment of endometriosis after laparoscopic surgery: results of a prospective, multicenter, randomized study. Fertil Steril 2002;77:684-692. PMID: 11937116.


20. Fraser IS. Bleeding arising from the use of exogenous steroids. Baillieres Best Pract Res Clin Obstet Gynaecol 1999;13:203-222. PMID: 10755038.


-
METRICS

-
- 16 Crossref
- 3,785 View
- 60 Download
- Related articles in Obstet Gynecol Sci
-
Estrogen Receptor Gene PvuII and XbaI Polymorphism in Patients with Endometriosis.2003 August;46(8)
A Case of Ruptured Abdominal Pregnancy Associated with Endometriosis.2004 July;47(7)
Treatment strategy for postoperative persistent pain of endometriosis.2005 August;48(8)