Comparison of International Ovarian Tumor Analysis ADNEX model and Ovarian-Adnexal Reporting and Data System with final histological diagnosis in adnexal masses: a retrospective study
Article information
Abstract
Objective
The International ovarian tumor analysis (IOTA)-Assessment of Different NEoplasias in the adneXa (ADNEX) model and the ovarian-adnexal reporting and data system (O-RADS) were developed to improve the diagnostic accuracy of adnexal masses in the preoperative period. This study aimed to evaluate the predictive values of both models in patients who underwent surgery for an adnexal mass at our hospital, based on the final pathological results.
Methods
This study included patients who underwent surgery for adnexal masses at our hospital between 2019 and 2021 and met the inclusion criteria. The IOTA ADNEX model and O-RADS scores were calculated preoperatively.
Results
Of the 413 patients, 295 were diagnosed with benign tumors and 118 were diagnosed with malignant tumors. The mean cancer antigen 125 (CA-125) levels for patients diagnosed with benign and malignant were 15.2 unit/mL and 72.5 unit/mL, respectively. According to the receiver operator characteristic analysis for serum CA-125 in postmenopausal and premenopausal patients, the cutoff value of 34.8 unit/mL had a sensitivity of 70.8% and specificity of 83.8% and 180.5 unit/mL had a sensitivity of 32.1% and a specificity of 92.7%, respectively (P<0.001). The sensitivity and specificity values of the IOTA ADNEX model and O-RADS were found as 78.8–48.3% and 97.9–93.5% respectively (P<0.001). There was moderate agreement between the IOTA ADNEX model and O-RADS (Kappa=0.53).
Conclusion
The IOTA ADNEX model has a similar specificity to the O-RADS in malignancy risk assessment, but the sensitivity of the IOTA ADNEX model is higher than that of the O-RADS. The IOTA-ADNEX model can help avoid unnecessary surgeries.
Introduction
Differential diagnosis of adnexal masses remains a compelling issue in gynecology. The preliminary diagnosis of adnexal masses determines the management strategy; therefore, an accurate preoperative evaluation of adnexal masses is crucial [1]. Transvaginal ultrasonography is routinely used to identify adnexal masses and discriminate between benign and malignant masses. Accurate discrimination between benign and malignant adnexal masses helps to determine the need for surgery at the most appropriate center and surgeon [2]. Follow-up or conservative management is more suitable for treating masses that are most likely to be benign. In contrast, patients with adnexal masses suspected of malignancy should be referred to a gynecological oncologist for better outcomes [3,4].
By 2020, approximately 313,959 new cases of ovarian cancer were diagnosed worldwide, resulting in 207,252 deaths. Ovarian cancer is the third most common gynecological malignancy and the second most common cause of death worldwide [5]. Most patients with ovarian cancer are diagnosed at advanced stages because of vague symptoms, such as abdominal swelling and low appetite, during the early stages of the disease. Patients diagnosed with advanced-stage ovarian cancer have a 5-year survival rate of <30.0%. In contrast, patients with early stage ovarian cancer have a 5-year survival rate of >92.4%. Early and accurate detection of ovarian cancer using noninvasive diagnostic methods such as ultrasonography improves patient survival rates [6].
With improvements in the image quality of ultrasonography machines, the number of patients diagnosed with adnexal masses has increased. Adnexal masses with a low malignancy risk can be followed up. Benign and malignant adnexal masses should be distinguished accurately using more sensitive and specific tests [7].
The American College of Radiology defined a classification system called the ovarian-adnexal reporting and data system (O-RADS) to standardize the reporting of sonographic findings and assess the malignancy risk of an adnexal mass. This scoring system is based on the maximum diameter of the lesion, number of locules, type of cyst (dermoid, endometrioma, or hemorrhagic), number of papillary projections, and presence of a solid component, ascites, or peritoneal nodules. This system categorizes adnexal masses into six groups: O-RADS 0–5. The O-RADS 0 category indicates incomplete evaluation; O-RADS 1 category refers to normal or physiological ovary; O-RADS 2 category refers to lesions that are most certainly benign with a risk of malignancy below 1.0%; O-RADS 3 category refers to lesions with a low risk of malignancy (1.0% to <10.0%); O-RADS 4 category refers to lesions with an intermediate risk of malignancy (10.0% to <50.0%); and O-RADS 5 category refers to lesions with a high risk of malignancy above 50.0% [8].
The International ovarian tumor analysis (IOTA) group developed the Assessment of Different NEoplasias in the adneXa (ADNEX) model using data from a study conducted on approximately 6,000 patients in 10 countries. This model was based on three clinical variables (age, serum cancer antigen 125 [CA-125] level, and oncology center) and six ultrasound variables (maximum lesion diameter, maximum solid component diameter, >10 locules, number of papillary projections, acoustic shadowing, and ascites). After applying the ADNEX model to a patient with an adnexal mass, the lesion was classified into five categories: benign, borderline, stage I invasive, stage II-IV invasive, and secondary metastatic tumors [9,10].
The aim of this study was to evaluate the O-RADS and IOTA ADNEX model scores of patients who had been diagnosed with and had undergone surgery for an adnexal mass in our hospital and to compare the O-RADS and IOTA ADNEX model systems’ accuracy of discrimination between benign and malignant adnexal masses based on the final pathological report.
Materials and methods
1. Ethics approval
This retrospective study was reviewed and approved by the Ethics Committee of Ankara Bilkent City Hospital (approval number: E2-21-818; September 20, 2021).
2. Study design and patients
This was a single-center, retrospective cohort study conducted at the Department of Obstetrics and Gynecology of Ankara Bilkent City Hospital, which is a tertiary referral center. A total of 413 patients were diagnosed with adnexal masses using ultrasonography and underwent surgery between September 2019 and September 2021. The inclusion criteria were as follows: 1) having undergone surgery for the presence of an adnexal mass, 2) being older than 18 years, and 3) having serum CA-125 levels and ultrasonographic morphology records of the adnexal mass. Patients younger than 18 years were excluded from the study because they were primarily admitted to pediatric surgery clinics rather than to obstetrics and gynecology clinics in our hospital. Patient scores were calculated using O-RADS and IOTA ADNEX model software. Subsequently, the diagnostic performance of these models was compared based on the final histological diagnosis.
3. Ultrasonographic examination
All included patients underwent transvaginal or transabdominal ultrasonography. A GE Healthcare Voluson S10 ultrasound device (GE Healthcare Technologies Inc., Chicago, IL, USA) was used by radiologists or gynecologists to evaluate patients with adnexal masses. When more than one adnexal mass was detected in the same patient, the mass with the most complex ultrasonographic morphology was selected to estimate the risk of malignancy.
4. Statistical analysis
Statistical analyses were performed using IBM Corporation SPSS version 22.0 (IBM Corporation, Armonk, NY, USA). The Kolmogorov-Smirnov test was used to analyze conformity to normal distribution. Descriptive statistics of continuous variables are shown as “mean±standard deviation” for those with normal distribution, and as “median (min-max value)” for those that do not. Categorical variables were compared using the chi-squared test or Fisher’s t-test. Continuous variables that were and were not normally distributed were compared using the independent sample t-test and the Mann-Whitney U test, respectively. Receiver operator characteristic (ROC) curve was applied to calculate and compare the areas under the curve (AUC) and determine the best cutoff values. Statistical significance for all tests was defined as P-value of less than 0.05. Kappa (κ) statistics were applied to assess the agreement between the O-RADS and IOTA ADNEX model scoring results for diagnosing malignancy. The κ values were interpreted according to the scale as follows: 0.01–0.20=poor agreement; 0.21–0.40=fair agreement; 0.41–0.60=moderate agreement; 0.61–0.80=good agreement; and 0.81–1.0=very good agreement.
Results
A total of 413 patients who met the inclusion criteria were included in this study. There were 295 (71.4%) benign and 118 (28.6%) malignant tumors. The mean patient age in this study was 48.7±14.8 years. The mean age of patients with benign versus malignant adnexal masses were 47.8±14.7 years and 51.1±14.9 years, respectively. Fifty-six patients (13.6%) were nulliparous and 357 (86.4%) were multiparous. The histopathological diagnosis was malignancy in 18 (32.1%) nulliparous patients and 100 (28.0%) of the multiparous patients. Of these patients, 231 (55.9%) were premenopausal and 182 (44.1%) were postmenopausal. The final histopathological diagnosis was malignancy in 53 (22.9%) premenopausal and 65 (35.7%) postmenopausal patients.
The risk of malignancy in adnexal masses increases with mass size. The median diameter of benign lesions was 90 mm (16–350 mm), while that of malignant lesions was 108.5 mm (29–385 mm) (P=0.006).
In adnexal masses, the risk of malignancy increases with the presence and growth of solid components. Ultrasonographic examination of the 413 patients revealed solid components in 166 patients (40.2%). While the pathological results of 66 patients (39.8%) were benign, those of 100 patients (60.2%) were malignant. The median solid components for benign and malignant masses were 15 mm (5–50 mm) and 25 mm (5–190 mm), respectively (P<0.001).
The distributions of ultrasonographic morphological features for the benign and malignant cases used for the IOTA ADNEX model and O-RADS are shown in Table 1.
The median serum CA-125 level in the 413 patients included in this study was 21.6 unit/mL (2–9,820 unit/mL). The median serum CA-125 level was 15.2 unit/mL (2–3,096 unit/mL) in patients with benign tumors and 72.5 unit/mL (5–9,820 unit/mL) in patients with malignant tumors (P<0.001). High serum CA-125 levels are associated with a high risk of adnexal mass malignancy. The cutoff values for serum CA-125 levels were calculated using the Youden index for postmenopausal and premenopausal patients. The cut off values of 34.8–180.5 unit/mL, sensitivity values of 70.8–32.1%, specificity values of 83.8–92.7%, AUC values of 0.847 (0.79–0.90) and 0.727 (0.65–0.80) in postmenopausal and premenopausal women, respectively (P<0.001) (Table 2, Fig. 1).

Evaluation of serum CA-125 level to predict malignancy of adnexal mass in postmenopausal and premenopausal patients by ROC analysis
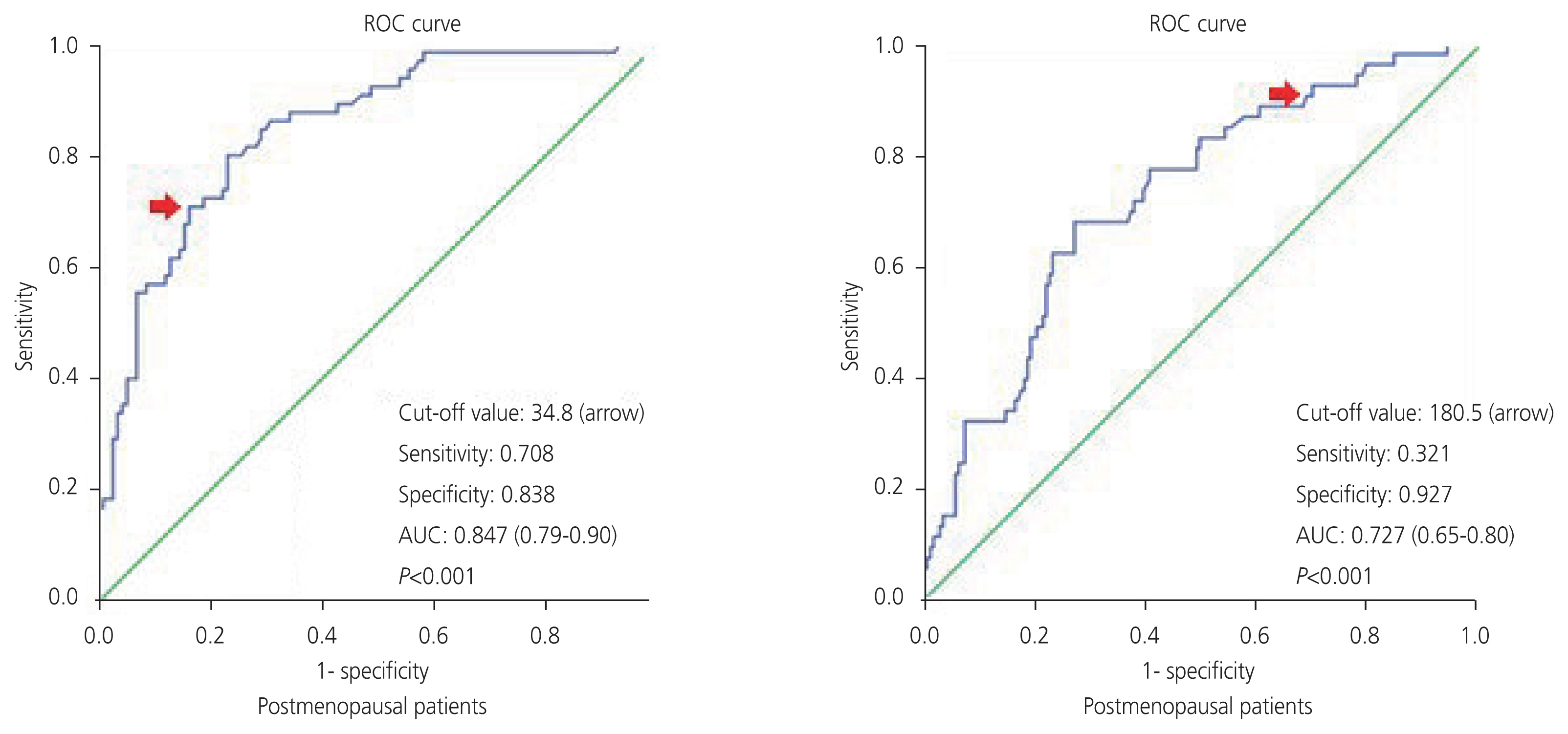
ROC curves of serum CA-125 to predict malignancy of adnexal mass in postmenopausal and premenopausal patients. ROC, receiver operator characteristic; AUC, areas under the curve; CA-125, cancer antigen 125.
At a cutoff value of 50.0% to predict malignancy preoperatively, the sensitivity of the IOTA ADNEX model was 78.8% and the specificity was 97.9% (P<0.001). At the same cutoff value, the sensitivity and specificity of the O-RADS model were 48.3% and 93.5%, respectively (P<0.001). Table 3 shows the statistical analysis of the IOTA ADNEX model and O-RADS classification system to predict malignant adnexal masses.

Statistical analysis of the IOTA ADNEX model and O-RADS classification system to predict malignant adnexal masses (at the 50% cut-off value)
The optimum cutoff value was found to be 49.7% when ROC analysis with the Youden index method was applied to the IOTA ADNEX model. At this cutoff value, the AUC was 0.957 (95% confidence interval [CI], 0.934–0.980), the sensitivity was 78.2%, the specificity was 80.0%, and the likelihood ratio was 38.29 (P<0.001).
Table 4 shows the relative risk ratios of the IOTA ADNEX model and O-RADS risk scoring systems for the estimation of malignancy risk in adnexal masses. The odds ratio for the IOTA ADNEX model was 179.1 (95% CI, 71.3–450.1) and 13.5 (95% CI, 7.5–24.4) for O-RADS risk scoring system (P<0.001).

Relative risk ratios of IOTA ADNEX model and O-RADS risk scoring systems for prediction of malignant adnexal masses
Based on the kappa index calculation, there was a moderate agreement between the IOTA ADNEX model and the O-RADS risk scoring system, which was statistically significant (κ=0.53; P<0.001).
Discussion
When an adnexal mass is detected on physical or ultrasonographic examination, the aim is to distinguish the mass as benign or malignant to aid in appropriate follow-up, which also includes referral to gynecological oncology. Ultrasonography-based models and tumor markers can be used for this differentiation.
Serum CA-125 has been the most frequently used tumor marker since its development as a preliminary test for adnexal masses [11]. Sensitivity and specificity of CA-125 as a tumor marker for the prediction of ovarian cancer were 61.0–90.0% and 71.0–93.0%, respectively [12–14]. Regarding ovarian cancers, the specificity and positive predictive value of CA-125 were higher in postmenopausal patients than in premenopausal patients [15]. According to the guidelines of The American College of Obstetricians and Gynecologists (ACOG), the cutoff values were 35 units/mL for postmenopausal patients and 200 units/mL for premenopausal patients who were suspected to have a malignant ovarian tumor. However, because these cutoff values were not determined based on high-quality evidence, the previous cutoff values were changed to a high CA-125 value for postmenopausal patients and a very high CA-125 value for premenopausal patients in the current ACOG guidelines [16]. In our study, the cut-off value for CA-125 was determined as 34.8 units/mL for postmenopausal patients, 180.5 units/mL for premenopausal patients and 49.6 units/mL for all patients (AUC with 95% CI: 0.847 [0.79–0.90], 0.727 [0.65–0.80], 0.776 [0.72–0.82]; sensitivity: 70.8%, 32.1%, 62.0% and specificity: 83.8%, 92.7%, 80.0%; respectively]. These results were consistent with those of previous studies [17,18]. However, this tumor marker shows poor performance in the differentiation of benign and malignant adnexal masses because it also increases in many benign conditions [19]. Therefore, it is reasonable to use CA-125 in combination with ultrasonography features rather than alone. Morphological ultrasound scoring systems have recently been developed to distinguish between benign and malignant ovarian masses. The IOTA ADNEX model and O-RADS scoring systems have been introduced as useful predictive tests to detect ovarian malignancy; however, head-to-head comparisons of these tests have not yet been performed. Therefore, we compared the IOTA ADNEX model and O-RADS scoring systems in this study.
The risk of ovarian malignancy algorithm (ROMA), which includes CA-125 and human epididymal protein (HE4), can be used to determine the malignancy risk in adnexal masses [20,21]. Because our study was retrospective and the patients did not have serum HE4 levels, other risk-scoring systems that calculate the risk of malignancy, such as ROMA, were not used.
In a study published by Van Calster et al. [22], according to the ROC analysis of the data used in developing the IOTA ADNEX model, the sensitivity and specificity percentages were 96.5% and 71.3%, respectively, regarding the differentiation of adnexal masses in terms of malignancy. We found a sensitivity of 78.8% and specificity of 97.9% for the IOTA ADNEX model for the preoperative diagnostic evaluation of adnexal masses when the cutoff value was 50.0%. In a study published by Jeong et al. [23], the optimal cutoff value for the IOTA ADNEX model was found to be 47.3%, with a sensitivity of 90.0% and specificity of 98.0%. In our study, we found results similar to the cutoff value of 49.7%. However, we found a sensitivity of 78.2% and specificity of 77.3%, which were lower than those reported in a previous study. Gynecologists might differentiate malignant adnexal masses from their benign counterparts more accurately and avoid unnecessary surgery by using this cutoff value in the IOTA ADNEX model.
In a study published by Cao et al. [24] in 2021, the sensitivity and specificity were 98.7% and 83.2%, respectively, for the O-RADS (at a 10.0% cutoff value). In our study, when O-RADS 4 and 5 lesions were suggested to be malignant preoperatively (at the same 10.0% cutoff value), the diagnostic accuracy, sensitivity, and specificity were 74.3% (95% CI, 0.698–0.784), 94.1% (95% CI, 0.882–0.976), and 66.3% (95% CI, 0.606–0.717), respectively. We found results with similar sensitivity but slightly lower specificity than those in the study published by Cao et al. [24]. When O-RADS 5 lesions were suggested to be potentially malignant (at the 50.0% cutoff value), the accuracy, sensitivity, and specificity were 80.3% (95% CI, 0.762–0.841), 48.3% (95% CI, 0.386–0.572), and 93.5% (95% CI, 0.900–0.960), respectively. At the 50.0% cutoff value, the O-RADS and IOTA ADNEX models had similar specificity, but the sensitivity of the IOTA ADNEX model was better than that of the O-RADS (diagnostic accuracy, sensitivity, and specificity for the IOTA ADNEX model: 92.2% [95% CI, 0.892–0.946], 78.1% [95% CI, 0.696–0.852], and 97.9% [95% CI, 0.956–0.992], respectively).
The IOTA ADNEX model provides results according to the malignancy type, unlike the O-RADS. In addition, the O-RADS does not determine the individual risk of each lesion, as the IOTA ADNEX model does. However, the IOTA ADNEX model requires several ultrasonographic features, specific software, and network connections to calculate the risk of malignancy. O-RADS requires much simpler ultrasonographic features, except color Doppler, than the IOTA ADNEX model, and can be performed using a simpler algorithm [8,9].
The main strength of our study is its relatively large population size. In addition, pathological specimens of all adnexal masses were obtained by surgery and not by biopsy. However, this study has some limitations. First, data selection bias may have affected the results of this study because of its retrospective nature. Second, ultrasonographic and pathological examinations were not performed by the same clinicians, which might have influenced the accuracy of diagnostic efforts during the evaluation of adnexal masses. Moreover, the data were acquired from a single tertiary referral hospital, which could have created bias owing to the sample distribution. Finally, the fact that the study included only operated adnexal masses, excluding adnexal masses suitable for follow-up, may explain why the sensitivity and specificity of the IOTA ADNEX model and O-RADS were slightly lower than those in the literature.
According to our study, although the IOTA ADNEX model has similar specificity to the O-RADS risk classification system for adnexal malignancy risk assessment, the sensitivity of the IOTA ADNEX model is higher than that of the O-RADS. The IOTA ADNEX model is a useful software based on ultrasonographic examination to differentiate benign from malignant adnexal masses and can help avoid unnecessary surgeries. Also, O-RADS is promising to be used for ultrasonography-based risk stratification of adnexal masses if the sensitivity can be increased with better classification of the O-RADS 4 group (10–50% risk of malignancy). Multicenter prospective studies with larger populations can delineate the differentiation performance of both the IOTA ADNEX model and the O-RADS risk evaluation system to predict adnexal/ovarian malignancies.
Notes
Conflict of interest
The authors declare no conflict of interests for this article.
Ethical approval
This retrospective study was reviewed and approved by the Ethics Committee of Ankara Bilkent City Hospital (approval number: E2-21-818, at September 20, 2021).
Patient consent
No informed consent was obtained from the patients because the study was retrospective.
Funding information
None.

