 |
 |
- Search
| Obstet Gynecol Sci > Volume 63(5); 2020 > Article |
|
Abstract
Objective
Exploiting their ability to differentiate into mesenchymal lineages like cartilage, bone, fat, and muscle, and to elicit paracrine effects, mesenchymal stem cells (MSCs) are widely used in clinical settings to treat tissue injuries and autoimmune disorders. One of accessible sources of MSC is the samples used for Papanicolaou (Pap) test, which is a cervical screening method for detecting potentially pre-cancerous and cancerous alterations in the cervical cells and to diagnose genetic abnormalities in fetuses. This study aimed to identify and isolate the stem cells from Pap smear samples collected from pregnant women, and to trace the origin of these cells to maternal or fetal tissue, and characterize their stem cell properties.
Methods
To investigate the possibility and efficiency of establishing MSC lines from the Pap smear samples, we were able to establish 6 cell lines from Pap smear samples from 60 pregnant women at different stages of gestation.
Results
The 3 cell lines randomly selected among the 6 established in this study, displayed high proliferation rates, several characteristics of MSCs, and the capacity to differentiate into adipocytes, osteocytes, and chondrocytes. Our study identified that the stem cell lines obtainable from Pap smear sampling were uterine cervical stromal cells (UCSCs) and had 10% efficiency of establishment.
Adult mesenchymal stem cells (MSCs) are defined as undifferentiated multipotent cells that are capable of self-renewal, differentiating into several distinct tissue lineages, such as bone, adipose tissue, and cartilage, and in vitro expansion. To date, many types of MSCs, derived from bone marrow (BM), adipose tissue, and amniotic fluid, show tremendous potential to be used for treating conditions ranging from organ failures to immunological diseases [1]. Although the therapeutic mechanisms of MSCs have not been fully explored, accumulated data demonstrate that these cells hold promise for clinical applications [2]. Various approaches have been tried for MSC-mediated tissue regeneration. As these cells have the potential for multilineage differentiation, and can secrete soluble factors that enhance cell survival and function, they can be used for patient-specific tissue regeneration without ethical restrictions, and are immune privileged. These special properties of MSCs have encouraged the researchers to find the best sources of these cells, preferably using noninvasive procedures. MSCs from various sources share many characteristics and generally meet the accepted criteria for MSCs, like multilineage differentiation potential, self-renewal capacity, expression of specific surface markers, and adherence to plastic surfaces. However, each of these cells also exhibit a set of unique and individualistic properties in their differentiation potentials, expression of specific surface markers, cytokine production, gene expression profiles, and ability to establish cell lines in vitro. Moreover, the ease with which biopsies are obtained differs between sources [3].
The Papanicolaou (Pap) smear test is a cervical screening method to detect potentially pre-cancerous and cancerous abnormalities in the cervix. At 1942, Dr. Papanicolaou first discovered a deformed nuclear morphology in benign cells collected by scraping the cervix, indicating their cervical and uterine cancer potentials [4]. In addition, because Pap smear samples from pregnant women contain fetal trophoblastic cells, this test is also used for non-invasive prenatal diagnosis since the 1950s [5]. Majority of the cells in Pap smear samples are epithelial cells, such as cervical cells from the mother and trophoblasts from the fetus. Because of the wide use and simplicity of the Pap smear test, the isolated human uterine cervical stromal cells (hUCSCs) have been recently proposed as a source of adult MSCs [6]. However, their reliability and accessibility for clinical applications are not clear and their potential as a source of MSC should be further explored by comparing it with well-established MSCs. This study is designed to verify the hUCSCs harvested from Pap smear as a reproducible and expandable source of MSCs for their clinical application. The Pap smear test is non-invasive and has no consequent complications, and thus can be performed on a wide population of women. These advantages have greatly facilitated the establishment of MSCs in the current study. Supported by our previous experience in identifying and characterizing potential sources of adult stem cells [7], we isolated and expanded hUCSC lines from Pap smear samples, and identified, assessed, and compared their MSC properties in terms of self-renewal and multilineage differentiation potentials compared with MSCs from amniotic-fluid. Importantly, although the Pap smear test was originally developed to collect cervical epithelial cells for cancer screening in women, it has been reported that the samples from pregnant women also contain fetus-derived cells [8]. We therefore investigated whether the isolated cell population contain fetus-derived trophoblast stem cells, using human leukocyte antigen (HLA)-G, from a family of non-classical HLA class I molecules, typically expressed in embryos, embryonic stem cells, and trophoblasts [9].
Pap smear samples were collected with informed consent, from 60 pregnant women at random, regardless of the length of their pregnancy. The collection procedure was approved by the Institutional Review Board of Korea University (KUGH16060-001). These samples were washed twice with phosphate-buffered saline (PBS) containing 100 U penicillin/streptomycin and centrifuged at 500 g for 10 minutes, and digested with 0.25% Trypsin/ethylenediaminetetraacetic acid (EDTA) (Hyclone, Waltham, MA, USA) for 30 minutes at 37°C. These were washed and centrifuged before the pellet was seeded into a 100 mm plate and incubated for 72 hours in low-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS), 100 U penicillin/streptomycin, and 1% L-glutamine at 37°C and 5% CO2. For expansion, adherent cells obtained from Pap smear samples, and amniotic fluidderived mesenchymal stem cells (AF-MSCs) were cultured in low-glucose DMEM containing 10% FBS, 100 U penicillin/streptomycin, 1% L-glutamine, 4 ng/mL basic fibroblast growth factor (R&D Systems, Minneapolis, MN, USA), and 50 μg/mL ascorbic acid (expansion medium). AF-MSCs were isolated and cultured in strict adherence to the guidelines issued by the Institutional Review Board of Korea University. These cells were previously confirmed to possess characteristics of MSCs, based on their differentiation, proliferation, and immunological phenotypes [7].
To determine the growth rates of hUCSCs and AF-MSCs, cells were seeded in 12-well plates at a density of 3×104 cells/well in expansion medium, cultured for 3 days, stained with 0.01% crystal violet solution, and de-stained with 10% acetic acid. Finally, absorbance at 600 nm was spectrophotometrically determined.
Adipogenic differentiation was induced using a previously described protocol [7]. Briefly, cells were seeded in 6-well plates at a density of 3×104 cells/well, and cultured until they reached 100% confluency. Thereafter, cells were grown for 7 days in high-glucose DMEM (Invitrogen) supplemented with 1 mM dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), 0.5 mM 3-isobutyl-1-methyl-xanthine (Sigma-Aldrich), 10 ng/mL recombinant human insulin (Sigma-Aldrich), 100 mM indomethacin (Sigma-Aldrich), and 10% FBS, to induce/maintain differentiation. After this, the cells were fixed in 10% formalin (Sigma-Aldrich) and stained with 2% (w/v) Oil Red O (Sigma-Aldrich) for 20 minutes at room temperature to detect oil droplets in the cytoplasm.
Osteogenic differentiation was induced according to a previously described protocol [7]. Briefly, cells were seeded in 6-well plates at a density of 3×104 cells/well, cultured in lowglucose DMEM (Gibco/Invitrogen) containing 10% FBS until they reached 100% confluency, and fed twice a week with osteogenic induction medium (high-glucose DMEM [Invitrogen] supplemented with 100 nM dexamethasone, 10 mM β-glycerophosphate, 0.2 mM ascorbate, and 10% FBS). Osteogenic differentiation was assessed by von Kossa staining.
To induce chondrogenic differentiation, cells were detached, transferred to a 15 mL polypropylene tube, pelleted via centrifugation at 200 g for 5 minutes, and cultured in highglucose DMEM supplemented with 0.1 M dexamethasone, 50 µg/mL ascorbic acid (Sigma-Aldrich), 100 µg/mL sodium pyruvate (Sigma-Aldrich), 40 µg/mL proline (Sigma-Aldrich) 10 ng/mL transforming growth factor-1 (R&D Systems), 50 mg/mL ITS premix (Gibco/Invitrogen), 6.25 µg/mL insulin, 6.25 µg/mL transferrin (Sigma-Aldrich), 6.25 ng/mL selenious acid (Sigma-Aldrich), 1.25 mg/mL bovine serum albumin (BSA; Sigma-Aldrich), and 5.35 mg/mL linoleic acid (SigmaAldrich). After 4 weeks of culture, cells were fixed in 4% paraformaldehyde and stained with Alcian Blue (Sigma-Aldrich).
RNA was isolated and purified using TRIzol (Invitrogen) according to the manufacturer’s instructions. cDNA was synthesized using Reverse Transcriptase II (Invitrogen). To amplify target genes, 25 ng cDNA was mixed with forward and reverse primers (Bioneer, Daejeon, Korea). The PCR conditions were as follows: 24-30 cycles of denaturation at 99°C for 30 seconds, annealing at 62°C for 30 seconds, and extension at 72°C for 30 seconds, followed by a final amplification step at 72°C for 10 minutes. The levels of target genes amplified by 30 cycles of PCR were within the linear range. Primer sequences are listed in Supplementary Table 1.
hUCSCs and AF-MSCs were trypsinized and transferred to fluorescence-activated cell sorting (FACS) tubes (BD Biosciences Clontech, Palo Alto, CA, USA) at a density of 1×106 cells/tube. Cells were rinsed twice with cold Dulbecco’s PBS containing 1% BSA (pH 7.4), and incubated with a primary antibody against CD29, CD31, CD34, CD44, CD45, CD73, CD90, or CD120a (BD Biosciences) or against human leukocyte antigen-G (HLA-G; Santa Cruz Biotechnology, Dallas, TX, USA) for 1 hour at 4°C. Thereafter, cells were washed twice with PBS containing 1% BSA, resuspended in 100 μL PBS containing 1% BSA and a fluorescein isothiocyanatelabeled secondary antibody diluted 1:100, and incubated for 40 minutes at 4°C. Finally, cells were washed twice with PBS containing 1% BSA and fixed in 4% paraformaldehyde for FACS analysis. To identify nonspecific signals, control cells were incubated with isotype-matched immunoglobulins.
hUCSCs and AF-MSCs were seeded in 6-well plates at a density of 100 cells/well, cultured for 14 days, washed twice with PBS, and fixed in 10% formalin for 20 minutes at room temperature. To visualize the colonies, cells were stained with 0.01% crystal violet solution for 20 minutes at room temperature, washed with deionized water, and air-dried. Colonies were typically 5-8 mm in diameter, and were scored macroscopically.
Karyotyping was performed by the Cytogenomic Services Facility of Samkwang Medical Laboratories. hUCSCs were cultured in expansion medium as described above. Cell were treated with 0.05 μg/mL colcemid (Gibco/Invitrogen) for 1-2 hour to block the dividing cells at metaphase. Chromosomes were visualized by G-banding. At least 100 metaphase cells were analyzed, and a minimum of ten cells were karyotyped per line.
To detect HLA-G, hUCSCs and AF-MSCs were incubated for 1 hour at 4°C with anti-HLA-G antibody (sc-21799; Santa Cruz Biotechnology) diluted in PBS containing 1% BSA, washed twice with PBS containing 1% BSA, and incubated for 40 minutes at 4°C with a fluorescein isothiocyanatelabeled secondary antibody diluted 1:100 in PBS containing 1% BSA. Nuclei were stained with DAPI diluted 1:1,000 in PBS containing 1% BSA.
We attempted to establish fetus-derived trophoblast cell lines by collecting the Pap smear sample from pregnant women. The cell samples were collected by centrifugation and seeded into cell culture dishes (Fig. 1A). Clusters of fibroblastlike cells with a homogenous morphology were observed in randomly selected areas after incubation for 72 hours (Fig. 1B). These adherent cells had a high proliferative potential in serum-containing medium. Isolated cells were stably sub-cultured for at least 12 passages (>40 days) (Fig. 1C). These cells became elongated and spindle-shaped upon repeated passages (Fig. 1B). Expression of HLA-G was detected by immunofluorescence staining in AF-MSCs, but not in our cell lines (Fig. 1D). This observation was supported by the FACS analysis, in which HLA-G was detected in AF-MSCs, and negatively in the cell lines isolated by us (Fig. 1D). Furthermore, the G-banding karyotype analysis showed normal female chromosomes (Fig. 1E). These findings indicate that the cells from Pap smear samples were derived from the cervix, which is in agreement with Eiró et al. [6]; trophoblast stem cells derived from the fetus could not be established in the current study.
We focused on establishment of hUCSC lines from cells derived from Pap smears of pregnant women. Of the 60 samples, only 6 hUCSC lines, including the one (Fig. 1, hUCSC #1) assessed earlier, were established. Similar to hUCSC #1, the two other hUCSC lines (#2, #3) showed high proliferation in serum-containing medium and could be stably subcultured for at least 12 passages (>40 days) (Fig. 2A). HLAG expression profiles of hUCSC #2 and #3 were similar to those of hUCSC #1 as observed in the immunofluorescence and FACS analysis (Fig. 2B). Karyotype of hUCSC #2 and #3 using G-banding showed normal female chromosomes (Fig. 2C). The immunophenotype of hUCSCs was analyzed by flow cytometry and it showed that AF-MSCs and hUCSC lines were negative for CD31, CD34, and CD45, indicating that they are not endothelial or hematopoietic in origin, but were positive for the MSC-specific markers CD29, CD44, CD73, CD90, and CD120a (Fig. 2D). In addition, both these MSCs abundantly expressed mesenchymal markers (Snail and Slug) and markers of extracellular matrix (fibronectin and syndecan) (Fig. 2E). The clonogenic and proliferation capacities of AF-MSCs and hUCSCs were compared by the colonyforming unit (CFU)-F assay. Colonies with a diameter larger than 5 mm were counted. The number of colonies formed did not significantly differ between hUCSCs and AF-MSCs or between the hUCSC lines (Fig. 2F).
For successful and efficient tissue regeneration using MSCs, their multilineage differentiation capacity must be preserved upon in vitro expansion. To investigate the adipogenic and osteogenic differentiation potentials of hUCSCs, cells at passage 4-6 were seeded at a density of 3×103 cells/cm2 and cultured in 10% FBS. At confluency, adipogenic and osteogenic differentiation were induced. Expected morphological changes and formation of neutral lipid droplets, as detected by Oil Red O, were observed at 1.5 weeks after induction of adipogenic differentiation (Fig. 3A). Reverse transcription polymerase chain reaction (RT-PCR) analysis demonstrated that adipocyte markers, LPL and aP2, were highly expressed by these cells upon induction of adipogenic differentiation (Fig. 3A). On osteogenic differentiation, von Kossa staining exhibited dark brown or black labeling, indicating mineralization of extracellular matrix in these cells (Fig. 3B). These cells expressed the osteocyte markers osteopontin and osteocalcin upon induction of osteogenic differentiation, as seen in RTPCR (Fig. 3B). The chondrogenic differentiation potential of hUCSCs was evaluated by culturing the cells in chondrogenic differentiation-inducing medium for 3-4 weeks. Glycosaminoglycans were detected by Alcian Blue staining and cells highly expressed the chondrocyte markers collagen type I and aggrecan, according to RT-PCR (Fig. 3C).
As mentioned above, from 60 pregnant women, we were able to establish only 6 hUCSC lines (Table 1). As Pap smear samples were randomly selected for this purpose, success or failure in establishment of hUCSCs were independent of donors’ age and gestational age. Furthermore, to study how the enzymatic digestion could affect the isolation efficiency, we digested only half of the fresh Pap smear samples with trypsin before starting the culture. Results suggest that the enzymatic digestion of Pap smear sample had no effect on the establishment of cell lines. We found the efficiency of establishing cell lines was 10%, regardless of donors’ age, duration of pregnancy and pre-treatment of Pap smear samples.
Rapidly advancing MSC-based therapeutic strategies have enormous potential for research and in regenerative medicine. The availability and accessibility of MSCs from various adult tissues and organs facilitate detailed studies of various stem/progenitor cell populations and their metabolic profiles in specific tissues, and promote the clinical applications of these cells. MSCs were first obtained from the stroma of BM, and BM is the most widely used source of stem cells for clinical trials [10]. Although BM-derived MSCs are considered as the gold standard, harvesting them from the BM frequently causes pain and harm to donors and patients [11]. Because of this, attempts were made to obtain MSCs from other sources. In the efforts to discover accessible sources of MSC and standardize the characteristics of these MSC, a variety of different tissue sources, including adipose tissue, the placenta, cord blood, peripheral blood, and amniotic fluid have been explored [12-15]. However, the following problems hinder the application of MSCs: i) donors often object to the harvesting of samples using needles, ii) it is difficult to collect the requisite number of homogeneous MSCs required for transplantation, iii) it is difficult to deliver the required dose of MSCs to the target site within the expected time frame, and iv) the occurrence of graft-versus-host disease is high and the survival rate is low among patients with nonmalignant disorders.
Although the properties of MSCs differ between different tissues and donors, MSCs originating from different tissues share common characteristics and generally meet the accepted criteria for MSCs, such as a self-renewal capacity, a fibroblast-like morphology, a multilineage differentiation potential, expression of typical surface markers such as CD29, CD44, and CD90, and lack of expression of lineage-specific markers such as CD14, CD34, and CD45 [16]. Wagner et al. [17] showed that the global gene expression patterns of MSCs derived from several tissues significantly differ. MSCs from tissues of mesodermal lineages (e.g., BM-derived MSCs) exhibit a propensity to express predominantly mesoderm-specific transcript homolog protein (MEST). On the other hand, expression of BMP antagonist 1 (also known as CKTSF1B1 and gremlin 1) and connective tissue growth factor (CTGF) are the highest in cord blood-derived MSCs. Expression of Ki-67, CDCA8, and CCNB2 are higher in adipose tissuederived MSCs than in BM-derived MSCs, implying that the proliferative potentials of the former are higher than those of the latter cells [18]. Distinct expression patterns of DLX5, which is an indicator of the osteogenic potential of stem cells, suggest that the differentiation potential of MSCs depends on the tissue source and the donor [19]. We adopted universally accepted criteria for defining MSCs to characterize fibroblast-like cell lines obtained from Pap smear samples. The proliferative and colony-forming capacities of hUCSCs and AF-MSCs were comparable (Figs. 1C and 2A), allowing extensive in vitro expansion of both. Our phenotypic analysis showed that hUCSCs were positive for typical MSC surface markers, such as CD29 (β-integrin), CD44 (hyaluronate receptor), CD71 (transferrin receptor), CD90 (Thy-1), and CD120a (tumor necrosis factor-α1 receptor), but negative for lineage-specific markers, such as CD31 (platelet endothelial cell adhesion molecule-1), CD34 (transmembrane phosphoglycoprotein), and CD45 (protein tyrosine phosphatase, receptor type, C) (Fig. 2D). Furthermore, we demonstrated the multilineage differentiation potential of hUCSCs by culturing them in specific differentiation-inducing conditions. MSCs from cord blood and placenta rarely differentiate into osteocytes, whereas their potentials to differentiate into adipogenic and chondrogenic lineages are comparable to those of BM-MSCs [19]. These findings indicate that differentiation capacity of hUCSCs is comparable with that of BM-derived MSCs, which are accepted as the gold standard, as trilineage differentiation of hUCSCs was successfully achieved (Fig. 3). These results strongly suggest that hUCSCs possess the unique features of MSCs (Fig. 4).
HLA-G, a nonclassical HLA class I molecule, has immunomodulatory actions, which disturb cytolysis, adhesion, and migration of natural killer cells [20]. Related studies revealed that HLA-G expression in MSCs differs between tissues, and that MSCs possess immunosuppressive properties and can inhibit the proliferation and function of major immune cell types, such as natural killer cells [21,22]. In the present study, the hUCSCs we isolated were HLA-G-negative, according to immunofluorescence and FACS analysis (Figs. 1 and 2). Hviid [23] reported that fetal trophoblasts, particularly the extravillous trophoblasts that invade the uterine wall and spiral arteries in the placenta, express HLA-G. By contrast, the maternal cells express HLA-ABC rather than HLA-G, which means that HLA analysis can be used to distinguish between fetal and maternal cells in undefined mixed cell populations. Regardless of the existence of fetal trophoblast cells, cells positive for HLA-G expression would depend on placental disorders, period of gestation, etc. [24-26]. Meanwhile, it is reasonable to assume that HLA-G-negative hUCSCs lack immunosuppressive properties; however, another study demonstrated that HLA expression is unrelated to inhibitory effects on T cell proliferation [19]. Therefore, the immunomodulatory effects of hUCSCs must be studied to resolve this question and to facilitate their broad clinical application.
At the beginning of this study, we aimed to establish the fetus-derived trophoblast stem cells from the Pap smears of pregnant women. However, the cells we isolated originated from maternal uterine cervix, but exhibited the following MSC properties, fibroblast-like morphology, self-renewal capacity in vitro, expression of MSC markers, and a multilineage differentiation potential. Our continued efforts resulted in isolation of stem cell with 10% efficiency of establishment, from the Pap smear samples of 60 pregnant women. The three hUCSC lines showed features comparable to those of AF-MSCs, generally considered as typical adult MSCs (Fig. 4). In conclusion, despite a low efficiency of establishment of hUCSCs, and the absence of fetal cells in the Pap smear samples, our results suggest that hUCSCs could be considered as a simple, safe, low-cost, and donor-specific source of cells for MSC-based therapy and regenerative medicine.
Acknowledgments
This work was supported by grants of the Korean Health Technology R&D project, Ministry of Health & Welfare, Republic of Korea (HI15C0810), the Ministry of Trade, Industry and Energy (MOTIE) and the Korea Institute for the Advancement of Technology (KIAT) (N0002405,2017), and School of Life Sciences and Biotechnology for BK21 PLUS, Korea University.
Notes
Supplementary materials
Supplementary Tables associated with this article can be
found online at https://doi.org/10.5468/ogs.20073.
Fig. 1.
Isolation of fibroblast-like cells from Papanicolaou (Pap) smear samples. (A) Method used to isolate cells from Pap smear samples. (B) Morphology of isolated cell line at passage 0 and 5 (scale bar=500 µm). (C) Accumulated population comparison with isolated cell line and amniotic fluid-derived mesenchymal stem cell (AF-MSC) upon passaging. (D) Immunofluorescence staining and fluorescence-activated cell sorting (FACS) analysis of human leukocyte antigen (HLA)-G in AF-MSCs and isolated cells (scale bar=200 μm). (E) Karyotype of isolated cell line determined by G-banding.
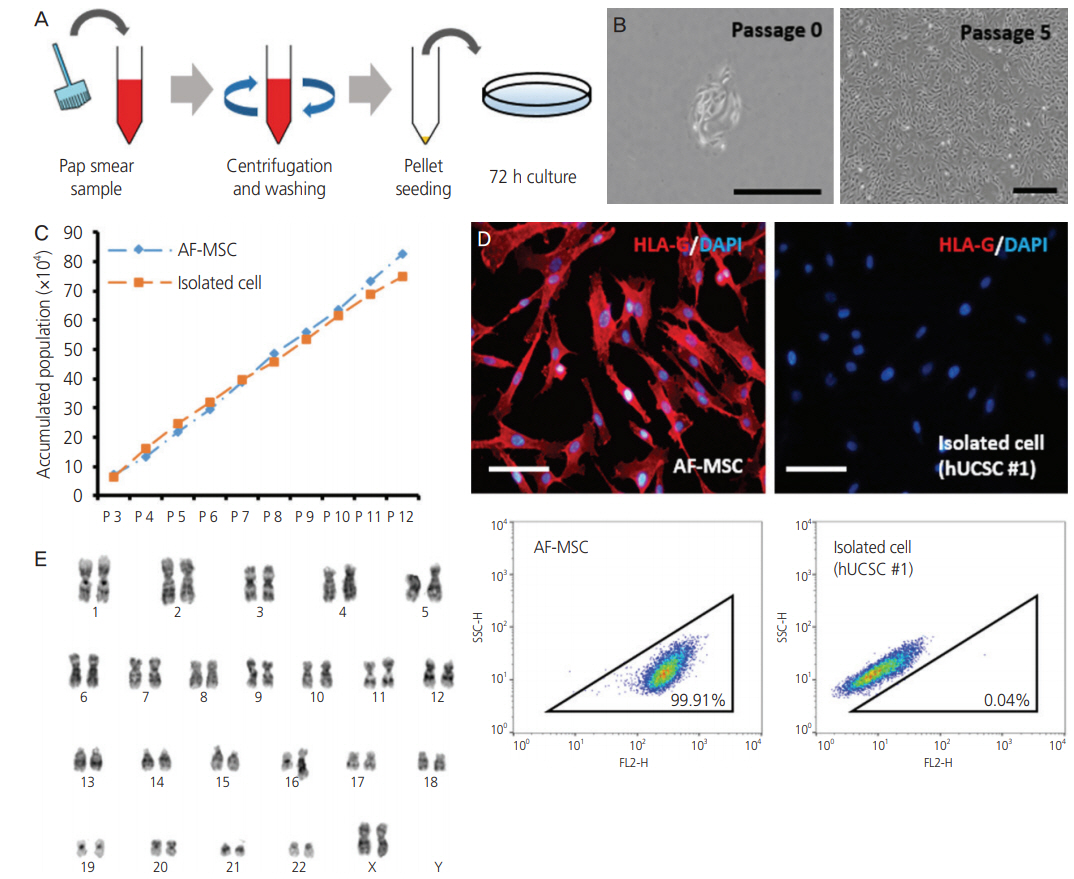
Fig. 2.
Characterization of human uterine cervical stromal cell (hUCSC) lines. (A) Accumulated population of hUCSCs and amniotic fluidderived mesenchymal stem cell (AF-MSC) upon passaging. (B) Immunofluorescence staining of human leukocyte antigen (HLA)-G in hUCSC line #2 and #3 (scale bar=200 μm). Fluorescence-activated cell sorting (FACS) analysis of HLA-G expression in hUCSC line #2 and #3. The percentages of HLA-G-positive cells are shown. (C) Karyotype of hUCSC line #2 and #3 determined by G-banding. (D) FACS analysis of the immunophenotypes of hUCSC lines (green). The isotype control is shown in purple. (E) mRNA expression of human MSC markers in hUCSC lines. (F) Colony-forming unit (CFU) assay investigating the self-renewal capacity of hUCSC lines.
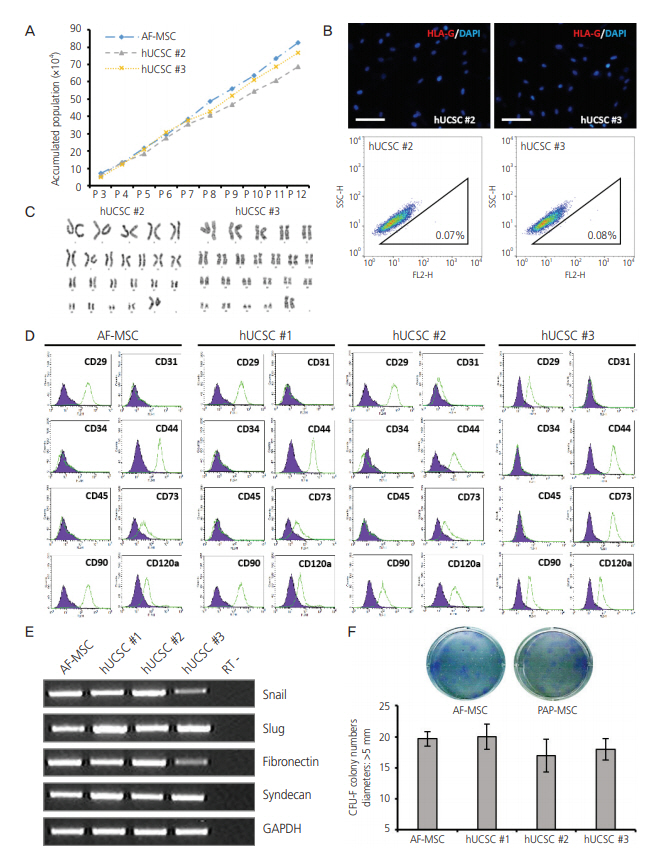
Fig. 3.
Differentiation of human uterine cervical stromal cells (hUCSCs). (A) Adipogenic differentiation of hUCSCs at passage 8. Differentiation was evaluated by Oil Red O staining and reverse transcription polymerase chain reaction (RT-PCR) analysis of adipocyte markers (LPL and aP2). (B) Osteogenic differentiation of hUCSCs at passage 8. Differentiation was evaluated by von Kossa staining and RT-PCR analysis of osteocyte markers (osteopontin and osteocalcin). (C) Chondrogenic differentiation of hUCSCs at passage 7. Differentiation was evaluated by Alcian Blue staining and RT-PCR analysis of chondrocyte markers (collagen I and aggrecan). Scale bar=200 µm. AF-MSC, amniotic fluid-derived mesenchymal stem cell; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
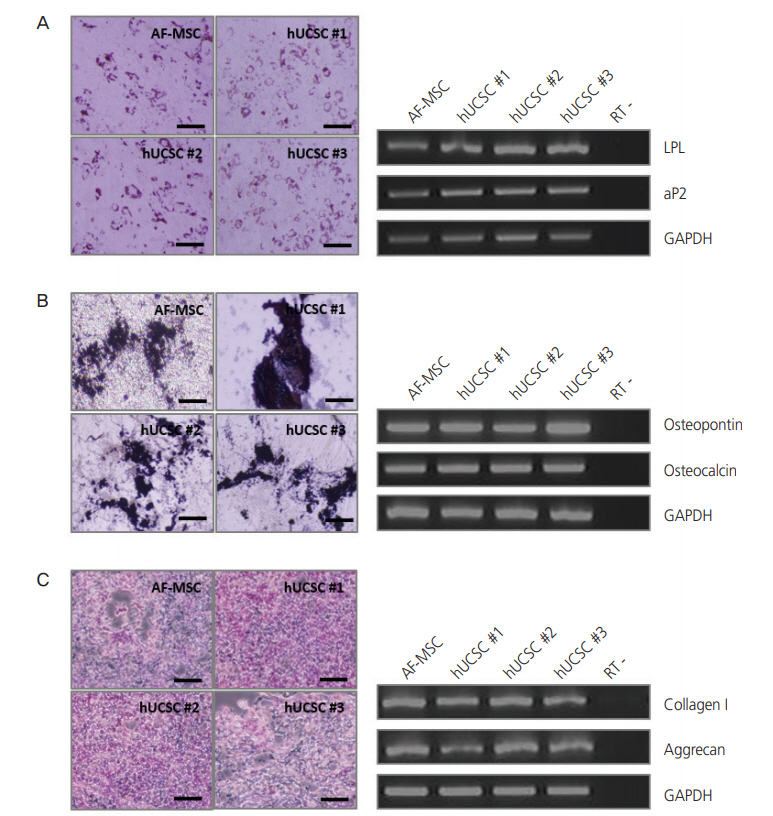
Fig. 4.
Establishment of human uterine cervical stromal cells (hUCSCs) from Papanicolaou (Pap) smear samples. MSC, mesenchymal stem cell.
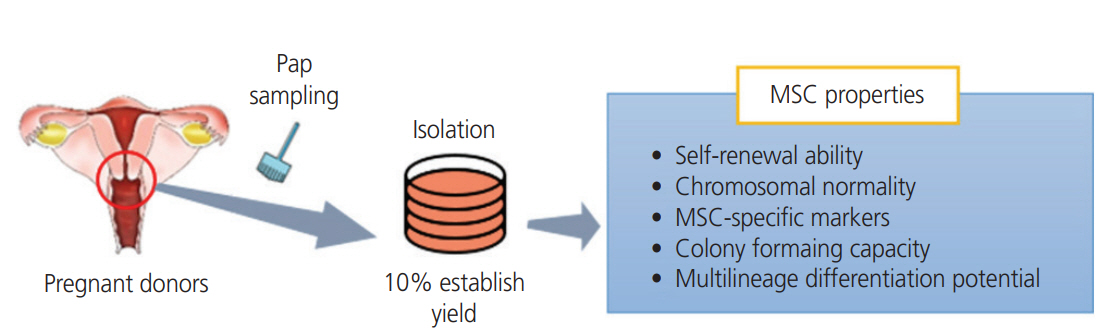
Table 1.
Isolation rate of Papanicolaou smear samples
References
1. Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant 2016;25:829-48.


2. Hwang IS, Kim HG, Jo DH, Lee DH, Koo YH, Song YJ, et al. Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells. Korean J Obstet Gynecol 2011;54:674-83.

3. Wu M, Zhang R, Zou Q, Chen Y, Zhou M, Li X, et al. Comparison of the biological characteristics of mesenchymal stem cells derived from the human placenta and umbilical cord. Sci Rep 2018;8:5014.




5. Pfeifer I, Benachi A, Saker A, Bonnefont JP, Mouawia H, Broncy L, et al. Cervical trophoblasts for non-invasive single-cell genotyping and prenatal diagnosis. Placenta 2016;37:56-60.


6. Eiró N, Sendon-Lago J, Seoane S, Bermúdez MA, Lamelas ML, Garcia-Caballero T, et al. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget 2014;5:10692-708.



7. Yoon BS, Moon JH, Jun EK, Kim J, Maeng I, Kim JS, et al. Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev 2010;19:887-902.


9. Rizzo R, Vercammen M, van de Velde H, Horn PA, Rebmann V. The importance of HLA-G expression in embryos, trophoblast cells, and embryonic stem cells. Cell Mol Life Sci 2011;68:341-52.



10. Klingemann H, Matzilevich D, Marchand J. Mesenchymal stem cells - Sources and clinical applications. Transfus Med Hemother 2008;35:272-7.



11. Fu WL, Zhou CY, Yu JK. A new source of mesenchymal stem cells for articular cartilage repair: MSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model. Am J Sports Med 2014;42:592-601.


12. Han J, Koh YJ, Moon HR, Ryoo HG, Cho CH, Kim I, et al. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood 2010;115:957-64.



13. Huang YC, Yang ZM, Chen XH, Tan MY, Wang J, Li XQ, et al. Isolation of mesenchymal stem cells from human placental decidua basalis and resistance to hypoxia and serum deprivation. Stem Cell Rev Rep 2009;5:247-55.



14. Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, Marx G, et al. Isolation of mesenchymal stem cells from G-CSFmobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant 2006;37:967-76.



15. Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from secondtrimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 2004;19:1450-6.



16. Horwitz EM, Le Blanc K, Dominici M, Mueller I, SlaperCortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy 2005;7:393-5.


17. Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005;33:1402-16.


18. Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem 2004;14:311-24.


19. Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med 2016;37:115-25.


20. Riteau B, Rouas-Freiss N, Menier C, Paul P, Dausset J, Carosella ED. HLA-G2, -G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J Immunol 2001;166:5018-26.


21. Götherström C, West A, Liden J, Uzunel M, Lahesmaa R, Le Blanc K. Difference in gene expression between human fetal liver and adult bone marrow mesenchymal stem cells. Haematologica 2005;90:1017-26.

22. Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol 2011;164:1-8.

23. Hviid TV. HLA-G in human reproduction: aspects of genetics, function and pregnancy complications. Hum Reprod Update 2006;12:209-32.



24. Yie SM, Taylor RN, Librach C. Low plasma HLA-G protein concentrations in early gestation indicate the development of preeclampsia later in pregnancy. Am J Obstet Gynecol 2005;193:204-8.


- TOOLS





















