Introduction
Misoprostol, a prostaglandin E1 analog, is commonly used in obstetrics/gynecology clinics for various purposes, including induction of labor and dilatation of the cervix prior to intrauterine procedures, including dilatation and curettage or hysteroscopy [1,2,3]. Although extremely rare, life-threatening anaphylactic shock can occur after misoprostol administration. This adverse event has previously only been reported in pregnant women, in one case during labor induction and in another during abortion [4,5]. Here we report a case of vaginal misoprostol as a cause of anaphylaxis prior to hysteroscopic myomectomy in a non-pregnant woman, a common indication of misoprostol application.
Case report
A para 2, 49-year-old woman presented with menorrhagia. Transvaginal sonography revealed 2 submucosal myomas of 3 cm each. A hysteroscopic myomectomy was planned after the administration of 3 doses of monthly gonadotrophin-releasing hormone agonists to decrease the size of the myomas. The patient's body mass index was 22.2 kg/m2. She was taking antihypertensive drugs and denied any known drug or nondrug allergies. She had undergone an aneurysm clipping for subarachnoid hemorrhage 18 months ago and laparoscopic cholecystectomy for acute cholecystitis 16 months ago in our hospital. On admission, her vital signs were normal, including a blood pressure of 142/80 mmHg, a pulse rate of 80 beats per minute, and a body temperature of 36.9°C. Misoprostol (Cytotec®, G.D. Searle LLC, Skokie, IL, USA) 400 μg was administered vaginally at 12:00 AM and 6:00 AM for cervical ripening prior to hysteroscopic myomectomy. Approximately 5 minutes after the second dose of vaginal misoprostol, the patient experienced uncontrolled shaking for 20 minutes; however, she did not complain of this because she thought it was just a common adverse effect of misoprostol. We routinely explain the possible adverse effects of misoprostol, including abdominal pain, vaginal bleeding, diarrhea, fever, and shivering to patients before administration of misoprostol. At 8:00 AM, the patient's body temperature was 39.0°C, and accompanying mild shivering was noted. Hydration with 300 mL of normal saline and 1 g of propacetamol hydrochloride (Denogan®, Yungjin Pharm. Co, Ltd., Seoul, Korea) was provided intravenously to control fever. At 9:30 AM, her body temperature decreased to 37.8°C, and whole-body plethora was noted. At 9:30 AM, her blood pressure decreased abruptly to 65/40 mmHg and pulse rate increased to 125 beats per minute immediately after the induction of general endotracheal anesthesia before the start of the hysteroscopic operation. Arterial cord blood gas analysis showed a pH of 7.27 and a base deficit of −1.6 mmol/L. Oxygen saturation (SaO2) was 98.9% (under mask O2 volume, 2 L), and the patient had a prolonged expiratory phase and a respiratory rate of 16 cycles/min, accompanied by generalized erythema, tachycardia, and hypotension. A physical examination revealed facial flushing, generalized edema, and a normal lung without evidence of oropharyngeal edema (Fig. 1). Her hemoglobin level was 11.8 mg/dL, and there was no evidence of excessive bleeding. Hydration with 300 mL of normal saline and a volume expander was provided, and ephedrine 5 mg was administered. After 30 minutes, she became normotensive, and her blood pressure slowly increased to 120/80 mmHg. Hysteroscopic myomectomy was performed successfully without retention of the expanding medium, the normal saline. Because of concern about intracranial hemorrhage, considering her history of aneurysm rupture and clipping surgery, a tentative diagnosis of cerebral hemorrhage was made. However, brain computed tomography, performed right after the operation, showed no evidence of hemorrhage or infarction. We also ruled out cardiogenic shock on the basis of a normal electrocardiogram; normal levels of cardiac markers including creatine kinase, CK-myocardial band, B-type natriuretic peptide, and cardiac troponin-I; and a normal echocardiogram. Chest X-ray and chest computed tomography revealed no evidence of pulmonary embolism, but interstitial pulmonary edema was noted (Fig. 2). We did not consider the possibility of an anaphylactic reaction to misoprostol and ruled out intracranial hemorrhage, pulmonary embolism, and cardiogenic shock because of the sudden unexplained hypotension. We could have administered epinephrine and diphenhydramine if we had made a tentative diagnosis of anaphylactic shock. Although we did not perform skin testing because of the patient's refusal, the patient had no allergies to any of the drugs administered before the anaphylactic shock other than the misoprostol because exactly the same drugs were used at the time of the aneurysm clipping and again at the time of cholecystectomy. A tentative diagnosis of anaphylactic shock to misoprostol was made based on case reports obtained after a PubMed search for unexplained shock performed 20 hours after the event [5,6,7,8]. The patient was in the intensive care unit for 24 hours until she was hemodynamically stable, and the generalized edema disappeared in 48 hours. On the fourth day, she was discharged without further adverse events; her condition was found to be normal without any complications at her 1- and 4-week follow-up visits.
Fig. 1
Clinical features of generalized edema caused by anaphylactic shock to intravaginal misoprostol. (A) Facial and neck edema. (B) Hand edema at 20 hours after the onset of anaphylactic shock.
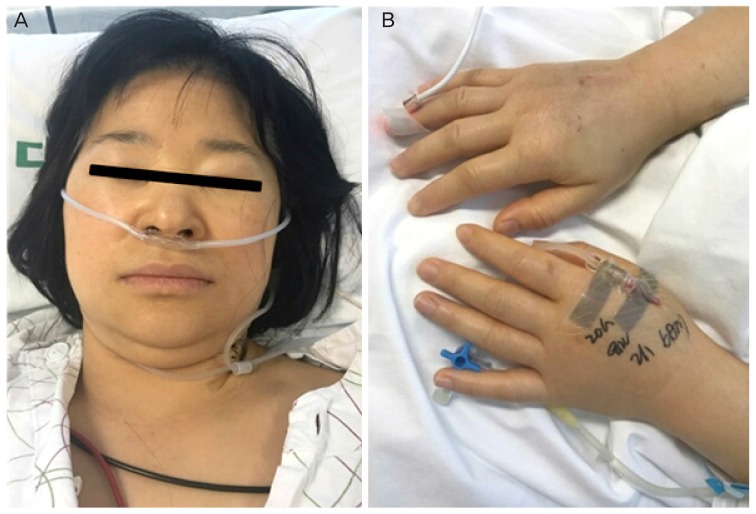
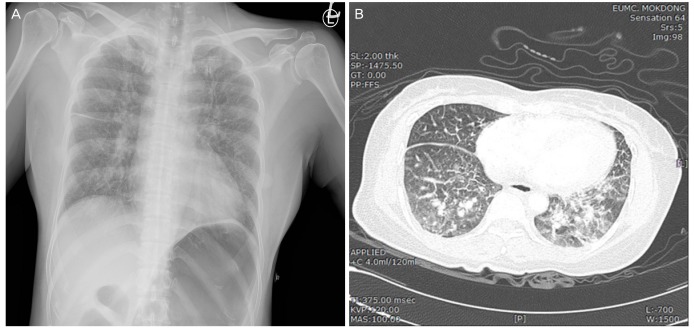
Fig. 2
Chest X-ray and chest computed tomography findings. (A) Chest X-ray showing interstitial pulmonary edema without cardiomegaly or pleural effusion. (B) An axial, contrast-enhanced computed tomography scan showing alveolar and interstitial pulmonary edema without evidence of pulmonary embolism.
Discussion
Among immunological reactions, the immediate type refers to a type 1 anaphylactic reaction, which is due to biologically active materials that are released from mast cells sensitized by specific immunoglobulin E antibodies. An allergic reaction occurs in the skin (urticaria/angioedema), respiratory and gastrointestinal tracts, or cardiovascular system, shortly after exposure to an allergen. The characteristic symptoms are shortness of breath, bronchospasms, soft-tissue swelling, edema, hypotension, itching, redness of the skin, wheezing, nausea, vomiting, diarrhea, cramps, and, in some cases, shock [9]. The first major symptoms of an anaphylactic reaction are urticaria and angioedema, and in severe anaphylaxis, pulmonary congestion and edema may be present. Anaphylactic symptoms associated with mucosal and parenchymal edema could be explained by both central and peripheral activation of mast cells in the lung parenchyma. Activation of mast cells increases vascular permeability, which in combination with systemic vasodilation, could lead to severe congestion [10].
According to studies about pharmacological mechanisms of misoprostol, it is generally known that misoprostol suppresses immune reactions that occur during the late phase of cutaneous allergic reactions and has a protective effect against allergic disease. However, contrary to previous studies, Babakhin et al. [11] showed misoprostol can block histamine release from basophils, and in several case reports about misoprostol-associated anaphylactic shock, it was shown that an allergic reaction could be caused by misoprostol. Misoprostol was initially approved for the prevention and treatment of peptic ulcers. However, misoprostol has been more frequently used in obstetrics/gynecology for cervical ripening and labor induction, although it is an off-label usage [1,2,3,4,5]. It is applied via the oral, sublingual, vaginal, and rectal routes, and compared to misoprostol administered orally, misoprostol administered via the sublingual and vaginal routes persists longer in the plasma [5]. Although a severe allergic reaction or anaphylactic shock due to misoprostol is very rare, obstetricians and gynecologists warn about the risks [7].
Madaan et al. [12] reported the case of a 32-year-old primigravida who presented at 12 weeks of gestation with a missed abortion. She experienced a severe hypersensitivity reaction beginning with symptoms such as shivering, an intense burning sensation, and feeling of warmth over the face, hands, and feet 20 minutes after intravaginal placement of 800 μg misoprostol. Further, when vaginal insert misoprostol was used in patients with early postpartum hemorrhage, symptoms such as tachycardia (heart rate, 120 bpm), high fever (39.8°C), and urticaria were noted [7]. Anaphylaxis and tachysystole started 4 hours after misoprostol administration in a 21-year-old woman who received oral misoprostol for post-date induction of labor at 41 weeks [6]. She initially complained of pruritus and flushing 20 minutes after misoprostol administration. Anaphylactic shock was also reported in a 17-year-old woman who received misoprostol in 2 separate doses for voluntary termination of pregnancy at 9 weeks of gestation in 2014 [7]. This anaphylactic shock was also noted 4 hours after the second dose of misoprostol. In the literature review, there were 2 reports of reactions to misoprostol in men. In a 63-year-old man, a repeated anaphylactoid reaction started 2 hours after the administration of oral misoprostol with diclofenac, a cyclooxygenase-2 inhibitor, and manifested as myocardial necrosis, rapidly dropping blood pressure, and facial flushing [8]. However, the man had already had an allergic reaction to rofecoxib, a cyclooxygenase-1 inhibitor; therefore, it was uncertain whether or not the anaphylactoid reaction was caused by misoprostol [8]. Shivering is a common adverse event in women who receive vaginal misoprostol; however, uncontrollable jerky shaking was the prodromal sign of anaphylactic shock in this patient and in the 63-year-old man who took misoprostol with cyclooxygenase-2. The report described the man saying that he could know the oncoming of the anaphylactoid reaction after experiencing the uncontrollable body shaking. A similar case of myocardial necrosis and anaphylactic shock in a 68-year-old man who took diclofenac with misoprostol was reported in French [13].
In the case of the subject of this case report, an allergic skin test for misoprostol could not be performed due to the patient's refusal. However, symptoms such as severe shivering immediately after misoprostol insertion, as well as generalized erythema, facial flushing, generalized edema, and hypotension that occurred later, correspond to an immediate-type immunologic reaction.
Other drugs that were administered to the patient included anesthetic drugs and denogan (paracetamol). The patient had received local and systemic anesthetic drugs several years before but had not experienced an allergic reaction. Paracetamol is a non-opioid analgesic and a non-steroidal anti-inflammatory drug that is used worldwide for analgesic and fever reduction purposes, and it is also known to cause hypotension as an allergic reaction. However, since most of these allergic reactions are associated with chronic use and overdose and acute side effects of this IV formulation have not been evaluated in many studies, the basis for such an association is weak [14,15]. Therefore, we believe that the anaphylactic shock was due to misoprostol rather than analgesics or paracetamol in this case.
We report the first case of anaphylactic shock to vaginal misoprostol in a non-pregnant woman. The possibility of anaphylactic shock should be considered in patients with sudden hypotension following misoprostol administration and prompt identification and management are crucial to prevent morbidity and mortality following anaphylactic shock to misoprostol.
Theoretically, we assume that a single dose of misoprostol could be safer than multiple doses for preventing sensitization to misoprostol. The risk of sensitization and the resulting anaphylactic shock to misoprostol can decrease with the use of a mechanical cervical dilator, such as laminaria, after the initial dose of misoprostol instead of a repeat dose for cervical dilation and ripening. Prompt identification along with determination of urinary and serum histamine levels and plasma tryptase levels as well as prompt management including the use of epinephrine and intravenous fluids are crucial to prevent morbidity and mortality following an anaphylactic shock to misoprostol. Adjunctive measures include airway protection and the use of antihistamines, steroids, and beta agonists. Patients taking beta-blockers may require additional measures [13].
We should keep in mind that even a frequently used drug can cause anaphylactic shock, which is a rare, life-threatening, emergent situation. Therefore, obstetricians and gynecologists should be well acquainted with the possibility of anaphylactic shock to misoprostol.























