Primary malignant melanoma of cervix and vagina
Article information
Abstract
Primary malignant melanoma (MM) accounts for 1% of all cancers, and only 3% to 7% of these tumors occur in the female genital tract. Data are limited with respect to the basis for treatment recommendations because of the rarity of MM. The overall prognosis of melanomas of the female genital tract is very poor. Two cases of MM of the female genital tract are presented. The first case is of a 70-year-old female patient who complained of left thigh pain and underwent magnetic resonance imaging that showed cervical cancer with involvement of the vagina, bladder, and parametrium, in addition to multiple bony metastases of the proximal femur, acetabulum, and both iliac bones. The second case is of a 35-year-old female patient who suffered from vaginal bleeding for 5 months, and she was diagnosed as having primary vaginal melanoma. The patient underwent radical surgery and two additional surgeries because of recurrence of cancer in both inguinal areas. After surgery, the patient received adjuvant immunotherapy, radiation therapy, and chemotherapy. In both the aforementioned cases, the pathologic diagnosis was made after immunohistochemical analysis, i.e., the tumor cells were stained with HMB-45 and S100, and were found to be positive for both immunostains.
Introduction
Primary malignant melanoma (MM) accounts for 1% of all cancers. MM can occur in any region of the mucosal sites (i.e., mucosal melanomas): the oral cavity, esophagus, anus, conjunctiva, or the gynecological tract. Primary vaginal melanoma accounts for less than 7% of all MMs, with less than 300 case reports worldwide, and 15 cases reported in the Republic of Korea [1]. Primary cervical melanoma is one of the rarest of these kinds of cancers; furthermore, it accounts for less than 2% of all MMs, with less than 50 cases reported in the literature [2]. Perhaps because of the rarity of primary cervical melanoma, there are no prospective, randomized, clinical trials assessing the effectiveness of various treatment options that have been undertaken in patients with melanomas of the female genital tract [3]. Thus, data are limited with respect to the basis for recommendation for the most efficacious treatment. The overall prognosis of MM of the female genital tract is very poor because it is usually diagnosed at an advanced stage and it spreads hematogenously at early stages [1].
Patients with MM of the female genital tract present with symptoms, such as vaginal bleeding, vaginal discharge, and vaginal mass [1]. Diagnosis is made by histological and immunological examination. Imaging studies are useful in identifying the primary site of malignancy and in determining extension of the mass. There are a few different staging systems that coexist for cervical MM and vaginal MM, respectively.
The recommended therapy for primary MM of the female genital tract is surgical excision. In cervical MM, radical surgery is preferred. Generally, radical hysterectomy with pelvic lymph node dissection and partial vaginectomy followed by radiation therapy (RT) delivered externally, such as external beam RT, or RT delivered internally, e.g., intracavitary RT, is most commonly used in treating gynecological cancers. On the other hand, in vaginal MM, wide local excision is the treatment of choice. It is highly debatable whether or not lymphadenectomy is essential. There are limited reports about the usefulness of adjuvant immunotherapy, RT, or chemotherapy.
Case report
1. Case 1
A 70-year-old female visited the emergency room with a left thigh pain. After physical evaluation, the patient underwent a left thigh magnetic resonance imaging (MRI) which showed a large cervical cancer with involvement of the vagina, bladder, and parametrium, in addition to multiple bony metastases of the proximal femur, acetabulum, and both iliac bones (Fig. 1).
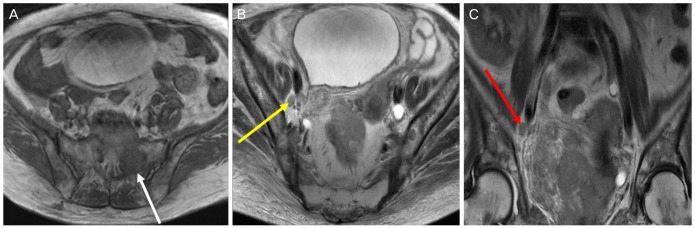
Magnetic resonance imaging findings. (A) About 8.6×7.3-cm enhancing cervical mass shows an invasion to the vaginal, bladder, and parametrium. It demonstrates mixed intermediate to subtle high signal intensity on T1WI. The white arrow indicates left sacral metastasis. (B) Right obturator lymph node metastasis is noted (yellow arrow). It shows heterogeneous intermediate to subtle high signal intensity on T2WI. (C) Right obturator lymph node metastasis and diffuse pelvic bone metastasis are noted (red arrow). It shows heterogeneous enhancement after contrast administration.
A gynecological examination showed an exophytic, hard, and pigmented mass, involving the cervix. A colposcopy-guided cervical biopsy was performed and that tissue was sent to the pathology laboratory for examination. These sheets of malignant cells revealed atypical features such as granular cytoplasm and prominent eosinophilic nucleoli (Fig. 2A, microscopic sections from the biopsy). These atypical cells showed a diverse morphology, from the spindle to epithelioid cells, and mixed in brownish pigments and abundant blood vessels. Next, these cells were subjected to immunohistochemical analysis, that is, they were stained with HMB-45 and S100. The antibodies against HMB-45 (melanosome) and S100 protein disclosed a diffused positive (Fig. 2B, C) reaction against tumor cells. Bearing in mind all the aforementioned findings, we diagnosed cervical MM.
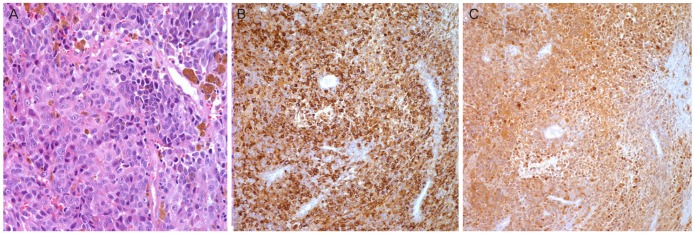
(A) Highly atypical sheets of malignant cells reveal sufficient granular cytoplasm and prominent eosinophilic nucleoli. The atypical cells show diverse morphology from the spindle to epithelioid cells, and mixed in brownish pigments and abundant blood vessels (H&E, ×400 ). (B) Immunohistochemical stains for HMB45 (×200) were done. Antibodies against HMB 45 (melanosome) disclose diffused positive reaction against tumor cells. (C) Immunohistochemical stains for S100 (×100) were done. Antibodies against S100 protein disclose diffused positive reaction against tumor cells.
The patient received focal argon laser treatment four times and went to a long-term acute care hospital to spend the rest of her life. She died two months after the initial diagnosis.
2. Case 2
A 35-year-old woman, para 2, who presented with a history of intermittent vaginal bleeding for five months, was referred to a tertiary healthcare institution. The patient was diagnosed with a vaginal MM by her private physician after a punch biopsy of a pinkish ulcerative lesion at the lower vagina. After a colposcopic examination, the following was noted: a 20-mm-sized erosive lesion at the 9 o'clock position of the lower vagina (Fig. 3A). After a positron emission tomography-computed tomography (CT), a mild fluorine-18-deoxyglucose uptake was shown at several lymph nodes in the left pelvic cavity.

(A) Colposcopic findings of primary malignant melanoma at vagina. It shows pinkish ulcerative lesion at lower vagina. (B) positron emission tomography-computed tomography (PET-CT) findings of recurrence of vaginal melanoma at left inguinal lymph node (white arrow). (C) PET-CT findings of recurrence of vaginal melanoma at right inguinal node (yellow arrow). (D) PET-CT findings of metastasis to multiple organs 37 months after the initial diagnosis, following three times of surgery and adjuvant therapy.
The patient further underwent a radical vaginectomy, a total abdominal hysterectomy, a bilateral salpingo-oophorectomy, and a left pelvic lymphadenectomy. A histologic examination revealed a 2.0×2.0-cm-sized nodular lesion with a depth of invasion of 700 µm; and a 0.4×0.2-cm-sized satellite lesion with a depth of invasion of 0.18 cm.
Immunohistochemically, the main lesion was stained with HMB-45 and S100; both of these immunostains were found to be positive. The lymph nodes, as well as surgical margins, were free from tumor. The patient underwent adjuvant immunotherapy with a high dose of interferon alpha2 beta. The patient was followed regularly in clinic and was disease-free for seven months.
Five months after completing adjuvant immunotherapy, a positron emission tomography-CT revealed an enlarged lymph node at the left inguinal area; an ensuing biopsy definitively determined that this condition was metastatic melanoma (Fig. 3B). The patient then underwent a left inguinal lymphadenectomy. Subsequently, the patient received adjuvant fractionated RT to the left inguinal area, with a weekly dose 48 Gy, for four times. After completion of the adjuvant fractionated RT, there was adjuvant chemotherapy with dacarbazine 1.8-mg body surface area applied at 3-week intervals, two times.
However, a later CT, which was taken for evaluation of the treatment response, revealed an enlarged lymph node at the right inguinal area, opposite to the one that was previously biopsied (left); and it was then proven to be a metastatic melanoma (Fig. 3C). The patient underwent a third operation, which was 14 months after the diagnosis and six months after her second operation. The patient underwent adjuvant fractionated RT to the right inguinal area, with a weekly dose 48 Gy, for four times.
Four months after the patient's third surgery, multiple lung metastases were found by an imaging and examination. The patient underwent palliative chemotherapy, eight times, with docetaxel 35 mg/m2 and a carboplatin area under curve 3 regimen; and chemotherapy, four times, with a cisplatin 30 mg and a vinblastine 1.8 mg regimen. Despite that chemotherapy, the disease progressed to the liver, left kidney, and duodenum (Fig. 3D). The patient expired by multiple organ failure, which was 37 months after the initial diagnosis.
Four months after her third surgery, multiple lung metastasis were found by imaging study. She underwent 8 times of palliative chemotherapy with docetaxel 35 mg/m2 + carboplatin area under curve 3 regimen and 4 times of chemotherapy with cisplatin 30 mg + vinblastine 1.8 mg regimen. Despite chemotherapy, disease progressed to liver, left kidney and duodenum. At last, she expired by multi organ failure, which was 37 months after the initial diagnosis.
Discussion
Primary MM of the female genital tract is very rare; furthermore, and most of the melanomas reported were of the vulva. In particular, primary cervical melanoma is one of the rarest tumors, with less than 50 cases reported in the literature [2]. Melanomas of the female genital tract are diagnosed at an advanced stage and they spread hematogenously at early stages through the vascular plexus around the female genital tract; thus, the overall prognosis is very poor [1]. The most recent National Cancer Database report indicated a 5-year survival of 11.4% for female genital tract melanoma [4].
Vaginal bleeding (63.6%), vaginal mass (15.9%), and vaginal discharge (15.9%) are common symptoms of female genital tract MM [15]. The gross appearance of female genital tract MM is pinkish in many cases, and it is commonly accompanied by ulceration compared to cutaneous MM that develops from a nevus [3]. In the first case, the primary cervical MM showed a cervical mass in the exophytic area, which was hard and pigmented, However in the second case, a pinkish ulcerative lesion was observed in the lower vagina. The gross findings of vaginal MM include various shapes of lesions, such as solitary, multifocal, superficial spreading, and a nodular shape [6].
Diagnosis of MM is made by histological and immunological examination using special immunostains such as those for S100 and HMB-45 [7]. To identify the primary site of cervical or vaginal MM, a thorough physical examination and imaging study are essential. Although there are few reports of metastasis from malignancies of kidney and breast, metastasis to the lower female genital tract is rare and mainly from adjacent organs; bladder and colorectum [89]. CT or MRI may be helpful to determine the extension, as well as the degree of cancer involvement. MRI can distinguish melanoma from other tumors because of a distinct signal pattern for melanin, i.e., high signal intensity on T1-weighted magnetic resonance images and low signal intensity on T2-weighted magnetic resonance images [10]. In both the cases, imaging studies were performed for detecting the primary site of malignancy instead of cystoscopy or colonoscopy.
There are two staging systems for primary MM of the cervix. Morrow and DiSaia have suggested the primary cutaneous melanoma staging system based on the thickness of the primary lesion, which is more clinically applicable to cervical MM compared to the International Federation of Gynecology and Obstetrics (FIGO) staging system for cervical cancer. However, the FIGO staging system for cervical cancer has been found to correlate well with the prognosis [11].
There are two coexisting staging systems for primary MM of the vagina as well. Recently, a few studies reported that the AJCC (American Joint Committee on Cancer) staging sytem based on the guidelines for cutaneous MM better reflects the overall prognosis for vaginal melanoma and is more adaptable than the FIGO staging for vaginal MM [13]. In these studies, invasion depth, margin, ulceration, and lymph node status were the major prognostic factors.
The overall prognosis for this kind of cancer is very poor. In a review of the literature for primary cervical melanoma, the 5-year survival rate for stage IA was 25% in the thirteen reported cases. The 5-year survival rate for stage II was 14%, and the 5-year survival rate for stages III and IV was 0% [10].
The recommended therapy for primary MM of the female genital tract is surgical excision. The therapy of choice for primary cervical MM is radical surgery, followed by focal radiotherapy; i.e., radical hysterectomy with pelvic lymph node dissection and partial vaginectomy, followed by RT such as external beam RT or intracavitary RT. However, from the clinical experience presented in the literature, MMs are generally considered to be radioresistant. Thus, irradiation is only useful in palliative therapy of a patient who is deemed unsuitable for radical surgery [12]. A patient can receive a single-agent or combination chemotherapy, using melphalan, vincristine, procarbazine, and bis-chloroethylnitrosourea, or a combination of bis-chloroethylnitrosourea, vincristine, and dimethyl triazeno imidazole carboxamide. However, the regression of tumors is transient [12]. Immunotherapy has also been introduced: the BCG (Bacille Calmette-Guérin) with interferon or interleukin-2. The results are not so promising [13].
As seen in the second case, surgery is the treatment of choice for vaginal MM. In a study of patients with vaginal MM at the University of Texas MD Anderson Cancer Center, the median survival was significantly different for women with vaginal MM who underwent surgery, compared to those who received other therapeutic modalities without surgery (24.3 to 34.4 vs. 8.7 months, respectively) [12]. However, there has been a debate with respect to the extent of surgery, i.e., recent reports have stated that wide local excision can achieve survival rates equivalent to radical surgery [11214]. For a routine lymphadenectomy, there is insufficient evidence for a low rate of regional lymph node metastasis [112]. Although sentinel lymph node biopsy is gaining in popularity due to low morbidity, further study is needed [15]. In spite of various kinds of recommendations, each patient's treatment should be individualized based on the disease status. For example, if local excision is impossible and there is suspicion of lymph node metastasis, radical surgery with lymphadenectomy may be a reasonable option.
There is limited evidence about the optimal adjuvant therapy for vaginal MM. Although MM is known to be resistant to radiotherapy and chemotherapy, recent studies with a small sample size showed that adjuvant treatment (including radiotherapy and chemotherapy) prolonged progression-free survival in vaginal MM patients who underwent wide local excision [1]. Immunotherapy with interferon is a widely used adjuvant therapy. Two agents (ipilimumab and vemurafenib) were approved by the US Food and Drug Administration in 2011 for the treatment of unresectable or metastatic cutaneous melanoma and they show promise with respect to the treatment of vaginal MM.
In conclusion, when patients visit the clinic with a mass or vaginal bleeding, primary MM should be considered as a differential diagnosis of other neoplasms. The pathologic diagnosis is usually confirmed using special stains and immunohistochemistry, if required. The treatment of choice for female genital tract MM is surgical excision and the prognosis is generally poor.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
